Abstract
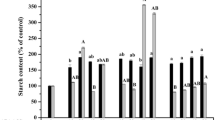
Embryogenic calli of sugarcane (Saccharum sp. hybrid, clone CP52-43), with somatic embryos in the late scutelar stage, were subjected to different treatments for increasing embryo tolerance to desiccation. The medium was supplemented with abscisic acid (ABA) (3.8 μM), jasmonic acid (JA) (4.7 μM) or a combination of them. A control treatment without growth regulators was also included. The embryos were encapsulated in alginate beads and dehydrated or not in sucrose (0.5 M). Thereafter, they were further dehydrated in chambers containing silicagel until the beads reached either 60% or 30% of water content (WC). Survival of encapsulated-dehydrated embryos was achieved only in the control and ABA treatment. ABA induced an increase in protein, polyamines, free proline levels and starch levels as a response to desiccation tolerance. JA treatment showed the lowest protein and polyamines levels and increased the starch content almost two-fold compared to the ABA treatment. The JA treatment induced high levels of 4-methylcatechol and the lowest levels of gallic acid. However, the ABA treatment increased gallic acid and p-coumaric acid content in the induction medium. Some differences were found in growth regulator free-medium in relation to the induction medium. JA is not effective in these desiccation processes. The mechanisms by which these two plant growth regulators act on the induction of tolerance to stress are presumably different.
Similar content being viewed by others
References
Adams CA & Rinne RW (1980) Moisture content as a controlling factor in seed development and germination. International Review of Cytology 68: 1-8
Alther S, Stirn S & Jacobsen HJ (1993) Inmunobiochemical analysis of a nuclear protein marker for regeneration potential in higher plants. J. Plant Physiol. 141: 415-422
Anandarajah K & McKersie BD (1990) Enhanced vigour of dry somatic embryos of Medicago sativa L. with increased sucrose. Plant Sci. 71: 261-266
Attree SM, Moore D, Sawhney VK & Fowke LC (1991). Enhanced maturation and desiccation tolerance of white spruce (Picea glauca (Moench) Voss) somatic embryos: Effects of a non-plasmolysing water stress and abscisic acid. Ann. Bot. 68: 519-525
Bates LS, Waldron RP & Teare LD (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39(1): 205-207
Bradford MM (1976)A rapid and sensitive method for the quantitation of microgram quanties of protein-dye binding. Anal. Bioch. 72: 248-254
Black M (1991) Involvement of ABA in the physiology of developing and mature seeds. In: Davies WJ & Jones HG (eds) Abscisic acid Physiology and Biochemistry Environmental Plant Biology series (pp 99-124). Bios Scientific Publishers
Carman JG (1988) Improved somatic embryogenesis in wheat by partial simulation of the in ovulo oxygen, growth regulator and dessication environments. Planta 175: 417-424
Carpita NC (1986) Incorporation of proline and aromatic aminoacid into cell wall of maize celeoptiles. Plant Physiol. 80: 660-666
Creelman RA & Nullet JE (1997) Biosynthesis and action of jasmonates in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48: 355-381
Cvikrová M, Hrubcová M, Eder J & Binarová P (1996) Changes in the levels of endogenous phenolics, aromatic monoamines, phenylalanine ammonia-lyase, peroxidase and auxin oxidase activities during initiation of alfalfa embryogenic and nonembryogenic calli. Plant Physiol. Biochem. 34(6): 853-861
Errea P, Treutter D & Feucht W (1992) Specificity of individual flavan-3-ols interfering with the grafting stress of apricots. Angewandte Botanik. 66: 21-24
Feucht W, Treutter D & Christ E (1992) The precise localization of catechins and proanthocyanidins in protective layers around fungal infections. Zeitschrift fur Pflanzenkrankheiten und Planzenschutz. 99: 404-413
Flores HE & Galston AW (1982) Analysis of polyamines in higher plants by High Performance Liquid Chromatography. Plant Physiol. 69: 701-706
Gutmann M, Von Aderkas P, Label P & Lelu MA (1996) Effects of abscisic acid on somatic embryo maturation of hybrid larch. J. Exper. Bot. 47(305): 1905-1917
Iida Y, Watabe K, Kamada H & Harada H (1992) Effects of abscisic acid on the induction of desiccation tolerance in carrot somatic embryos. J. Plant Physiol. 140: 356-360
Ingram J & Bartels D (1996) The molecular basis of dehydration tolerance in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47: 377-403
Kitto SL & Janick J (1985) Hardening treatments increase survival of synthetically-coated asexual embryos of carrot. J. Am. Soc. Hort. Sci. 110: 283-286
Lecouteux C, Lai FM & McKersie BD (1993) Maturation of somatic embryos by abscisic acid, sucrose and chilling. Plant Sci. 94: 207-213
Mace ME & Bell AA (1981) Fravonol and terpenoid aldehyde synthesis in tumours asociated with genetic incompatibility in a Gossypium hirsutum χ G. Gossypioides hybrid. Can. J. Bot. 59: 951-955
Minocha R, Kvaalen H, Minocha SC & Long S (1993) Polyamines in embryogenic cultures of Norway spruce (Picea abies) andRed spruce (Picea rubens). Tree Physiol. 13: 365-377
Murashige T & Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco cultures. Physiologia Plantarum. 15: 473-497
Nieves N, Lorenzo JC, Blanco MA, González JL, Peralta H, Hernández M, Santos R, Concepció n O, Borroto E, Borroto CG, Tapia R, Martínez M & Fundora Z (1998) Artificial endosperm by zygotic embryos of Cleopatra tangerine (Citrus reshni Hort ex Tan). A model by somatic embryos emcapsulation. Plant Cell Tiss. Org. Cult. 54(2): 77-83
Pitel JA, Yoo BY, Klimaszewska K & Charest P (1992) Changes in enzyme activity and protein patterns during the maturation phase of somatic embryogenesis in hybrid larch (Larix $#x03C7; eurolepis). Can. J. For. Res. 22: 553-560
Santanen A & Simola LK (1992) Changes in polyamine metabolism during somatic embryogenesis in Picea abies. J. Plant Physiol. 140: 475-480
Senaratna T, McKersie BD & Bowley SR (1990) Artificial seed of alfalfa (Medicago sativa L.). Induction of desiccation tolerance in somatic embryos. In Vitro Cell Ded. Biol. 26: 85-90
Takahata Y, Brown DCW, Keller WA & Kaizuma N (1993) Dry artificial seed and desiccation tolerance induction in microsporederived embryos of broccoli. Plant Cell Tiss. Org. Cult. 35: 121-129
Tapia R, Castillo R, Nieves N, Blanco MA, Gonzalez JL, Sanchez M & Rodriguez Y (1999) Inducció n, maduració n y encapsulació n de embriones somáticos de cañ a de azú car (Saccharum sp. híbrido) var. CP 5243. Biotecnología Aplicada. 16(1): 20-23
Tetteroo FAA, Hoekstra FA & Karssen CM (1995) Induction of complete desiccation tolerance in carrot (Daucus carota) embryoids. J. Plant Physiol. 145: 349-356
Timbert R, Barbotim JN & Thomas D (1996) Enhancing carrot somatic embryos survival during slow dehydration, by encapsulation and control of dehydration. Plant Science 120: 215-222
Yemm EW & Willis AJ (1966) The estimation of carbohydrates in plant extracts by Antrone. Biochem. J. 57: 508-514
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nieves, N., Martínez, M.E., Castillo, R. et al. Effect of abscisic acid and jasmonic acid on partial desiccation of encapsulated somatic embryos of sugarcane. Plant Cell, Tissue and Organ Culture 65, 15–21 (2001). https://doi.org/10.1023/A:1010699532641
Issue Date:
DOI: https://doi.org/10.1023/A:1010699532641