Abstract
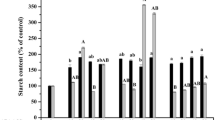
Polyethylene glycol 4000, mannitol and sorbitol were tested as supplements to a liquid Finer and Nagasawa medium-based histodifferentiation/maturation medium, FNL0S3, for soybean (Glycine max L. Merrill) somatic embryos of `Jack' and F138 or `Fayette'. Significant differences were found among types and levels of osmotica for their influence on quantity of mature embryos recovered, and on germination (root and shoot emergence) and conversion of embryos into plants. Supplementation of FNL0S3 with 5% polyethylene glycol or 1.5% sorbitol improved germination frequencies without limiting embryo histodifferentiation. Supplementation with 3% sorbitol resulted in a 9-fold increase in germination frequencies and a 13-fold increase in conversion frequencies of `Fayette' and `Jack' embryos. However, these improvements were accompanied by a significant, 22% reduction in fresh weight of mature embryos. Overall, 3% sorbitol was found to be the best of the osmotic supplements tested, resulting in 51 ± 5% conversion frequency (mean ± SE), as compared to 4 ± 1% in the control. Supplementation of FNL0S3 with 3% mannitol did not improve embryo maturation.
Similar content being viewed by others
References
Attree SM & Fowke LC (1993) Embryogeny of gymnosperms: advances in synthetic seed technology of conifers. Plant Cell Tiss. Org. Cult. 35: 1–35
Attree, SM, Moore D, Sawhney VR & Fowke LC (1991) Enhanced maturation and desiccation tolerance of white spruce (Picea glauca [Moench.] Voss) somatic embryos: Effects of a non–plasmolysing water stress and abscisic acid. Ann. Bot. 68: 519–525
Bailey MA, Boerma HR & Parrott WA (1993a) Genotype effects on proliferative embryogenesis and plant regeneration of soybean. In Vitro Cell. Dev. Biol. 29P: 102–108
Bailey MA, Boerma HR & Parrott WA (1993b) Genotype–specific optimization of plant regeneration from somatic embryos of soybean. Plant Sci. 93: 117–120
Blackman SA, Obendorf RL & Leopold AC (1992) Maturation proteins and sugars in desiccation tolerance of developing soybean seeds. Plant Physiol. 100: 225–230
Egli DM (1990) Seed water relations and the regulation of the duration of seed growth in soybean. J. Exp. Bot. 41: 243–248
Finer JJ & McMullen MD (1991) Transformation of soybean via particle bombardment of embryogenic suspension culture tissue. In Vitro Cell. Dev. Biol. 27P: 175–182
Finer JJ & Nagasawa A (1988) Development of an embryogenic suspension culture of soybean [Glycine max (L.) Merrill]. Plant Cell Tiss. Org. Cult. 15: 125–136
Finkelstein RR & Crouch ML (1986) Rapeseed embryo development in culture on high osmoticum is similar to that in seeds. Plant Physiol. 81: 907–912
Fischer W, Bergfeld R & Schopfer P (1987) Induction of storage protein synthesis in embryos of mature plant seeds. Naturwissenschaften 74: 86–88
Fujii JAA, Slade D, Olsen R, Ruzin & Redenbaugh (1990) Alfalfa somatic embryo maturation and conversion to plants. Plant Sci. 72: 93–100
Hammatt N & Davey MR (1987) Somatic embryogenesis and plant regeneration from cultured zygotic embryos of soybean (Glycine max L. Merr.). J. Plant Physiol. 128: 219–226
Kermode A (1995) Regulatory mechanisms in the transition from seed development to germination: interactions between the embryo and the seed environment. In: Kigel J & Galili (eds) Seed Development and Germination (pp 273–332). Marcel Dekker, Inc., New York
Komatsuda T, Lee W & Oka S. (1992) Maturation and germination of somatic embryos as affected by sucrose and plant growth reguators in soybeans Glycine gracilis Skvortz and Glycine max (L.) Merr. Plant Cell Tiss. Org. Cult. 28: 103–113
Mhaske VB, Chengalrayan K & Hazra S (1998) Influence of osmotica and abscisic acid on triglyceride accumulation in peanut somatic embryos. Plant Cell Rep. 17: 742–746
Murashige T & Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15: 473–497
Obendorf RL, Dickerman AM, Pflum TM, Kacalanos MA & Smith ME (1998) Drying rate alters soluble carbohydrates, desiccation tolerance, and subsequent seedling growth of soybean (Glycine max L. Merrill) zygotic embryos during in vitro maturation. Plant Sci. 132: 1–12
Parrott WA, Dryden G, Vogt S, Hildebrand DF, Collins GB & Williams EG (1988) Optimization of somatic embryogenesis and embryo germination in soybean. In Vitro Cell. Dev. Biol. 24: 817–820
Ranch JP, Oglesby L & Zielinski AC (1985) Plant regeneration from embryo–derived tissue cultures of soybean. In Vitro Cell Dev. Biol. 21: 653–658
Saab IN & Obendorf RL (1989) Soybean seed water relations during in situ and in vitro growth and maturation. Plant Physiol. 89: 610–616
Samoylov VM, Tucker DM & Parrott WA (1998a) Soybean embryogenic cultures: the role of sucrose and nitrogen content on proliferation. In Vitro–Plant 34: 8–13
Samoylov VM, Tucker DM, Thibaud–Nissen F & Parrott WA (1998b) A liquid–medium–based protocol for rapid regeneration from embryogenic soybean cultures. Plant Cell Rep. 18: 49–54
SAS Institute Inc: SAS user's guide statistics, Release 6.03, Cary, NC (1988)
Stoop JMH, Williamson JD & Pharr DM (1996) Mannitol metabolism in plants: a method for coping with stress. Trends Plant Sci. 1: 139–144
TeKrony DM, Egli DB, Balles J, Pfeiffer T & Fellows RJ (1979) Physiological maturity in soybean. Agron. J. 71: 771–775
Trick HN, Dinkins RD, Santarem ER, Di R, Samoylov VM, Meurer C, Walker D, Parrott WA, Finer JJ & Collins GB (1997) Recent advances in soybean transformation. Plant Tiss. Cult. Biotechnol. 3: 9–26
Wakui K, Takahata Y & Kaizuma N (1999) Scanning electron microscopy of desiccation–tolerant and–sensitive microsporederived embryos of Brassica napus L. Plant Cell Rep. 18: 595–600
Wright MS, Launis KL, Novitzky R, Duesing JH & Harms CT (1991) A simple method for the recovery of multiple fertile plants from individual somatic embryos of soybean [Glycine max (L.) Merrill]. In Vitro Cell. Dev. Biol. 27P: 153–157
Xu N, Coulter KM & Bewley JD (1990) Abscisic acid and osmoticum prevent germination of developing alfalfa embryos, but only osmoticum maintains the synthesis of developmental proteins. Planta 182: 382–390
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Walker, D.R., Parrott, W.A. Effect of polyethylene glycol and sugar alcohols on soybean somatic embryo germination and conversion. Plant Cell, Tissue and Organ Culture 64, 55–62 (2001). https://doi.org/10.1023/A:1010601402098
Issue Date:
DOI: https://doi.org/10.1023/A:1010601402098