Abstract
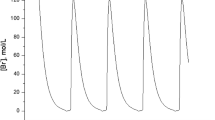
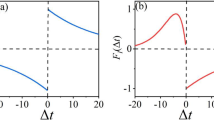
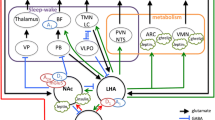
Much evidence indicates that the pineal gland and thesuprachiasmatic nucleus (SCN) are the primary pacemakers in the housesparrow, Passer domesticus. The interactions between the pineal andSCN predicted by the neuroendocrine loop model indicates that uncouplingwould cause the two oscillators to damp out in constant darkness. Basedupon the original neuroendocrine loop model, a mathematical frameworkof the house sparrow circadian regulatory organization that incorporatesdamping and co-inhibitory coupling has been formulated. The proposedmodel clearly indicates that two coupled oscillators must be 180°out of the phase for sustaining oscillations. From damping coefficients,which can be determined from experimental data, other parameters suchas external stimuli (interaction coefficient) and characteristicfrequencies can then be computed. Based upon earlier studies and simulations,we conclude that the sparrow pineal gland dampens more rapidly than does theSCN, suggesting that the SCN are probably more important in sparrowsthan previously thought. The model also provides the explanations ofendogenous circadian period (tau) alteration. Finally, we extend this modelto other avian and to mammalian circadian systems. We suggest that avianand mammalian circadian systems may differ in damping coefficients ofpineal glands and the degree of SCN dominance.
Similar content being viewed by others
References
Cassone, V.M. and Menaker, M.: Is the avian circadian system a neuroendocrine loop?, J. Exp. Zool. 232 (1984), 529-549.
Gaston, S. and Menaker, M.: Pineal function: the biological clock in sparrows?, Science 160 (1968), 1125-1127.
Binkley, S., Kluth, E. and Menaker, M.: Pineal function in sparrows: circadian rhythms and body temperature, Science 197 (1971), 1181-83.
Zimmerman, N.M. and Menaker, M.: Neural connections of sparrow pineal: role in circadian control of activity, Science 190 (1975), 477-479.
Zimmerman, N.M. and Menaker, M.: The pineal gland, a pacemaker within the circadian system of the house sparrow, Proc. natn. Acad. Sci. U.S.A. 76 (1979), 999-1003.
Murakami, N., Nakamura, H., Nishi, R., Marumoto, N. and Nasu, T.: Comparsion of circadian oscillation of melatonin release in pineal cells of house sparrow, pigeon and Japanese quail using cell perfusion system, Brain Res. 651 (1994), 209-214.
Takahashi, J.S., Hamm. H. and Menaker, M.: Circadian rhythms of melatonin release from individual superfused chicken pineal glands in vitro, Proc. natn. Acad. Sci. U.S.A. 77 (1980), 2319-2322.
Zatz, M.: Light and norepinephrine similarly prevent damping of the melatonin rhythm in cultured chick pineal cells: regulation of coupling between the pacemaker and overt rhythm, J. Biol. Rhythm 6(2) (1991), 137-148.
Cassone, V.M. and Menaker, M.: Sympathetic regulation of the chicken pineal rhythms, Brain Res. 289 (1983), 129-135.
Turek, F.W., McMillan, J.P. and Menaker, M.: Melatonin: effects on circadian locomotor rhythm in sparrows, Science 194 (1976), 1441-1443.
Ebihara, S. and Kawamura, K.: The role of pineal organ and the suprachiasmatic nucleus in the control of circadian locomotor rhythms in the Java sparrow (Padda oryzivora), J. Comp. Physiol. A. 141 (1981), 207-214.
Heigl, S. and Gwinner, E.: Synchronization of circadian rhythms of house sparrows by oral melatonin: effects of changing period, J. Biol. Rhythms Sep. 10(3) (1995), 225-233.
Lu, J. and Cassone, V.M.: Daily melatonin administration synchronizes circadian pattern of brain metabolism and behavior in pinealectomized house sparrow (Passer domesticus), J. Comp. Physiol. A. 173 (1993), 775-782.
Cassone, V.M. and Moore, R.Y.: Retinohypothalamic projection and suprachiasmatic nucleus of the house sparrow (Passer domesticus), J. Comp. Neurol. 266 (1987), 171-182.
Takahashi, J.S. and Menaker, M.: Role of the suprachiasmatic nucleus in the circadian system of the house sparrow, J. Neurosci. 2 (1982), 815-828.
Cassone, V.M., Forsyth, A.M. and Woodlee, G.M.: Hypothalamic regulation of circadian noradrenergic input to the chick pineal gland, J. Comp. Physiol. A. 167 (1990), 187-192.
Cassone, V.M.: Circadian variation of [14C]2-deoxyglucose uptake within the suprachiasmatic nucleus of the house sparrow (Passer domesticus), Brain Res. 459 (1988), 178-182.
Lu, J. and Cassone, V.M: Pineal regulation of 2-[14C]deoxyglucose uptake and 2-[125I]iodomelatonin binding in visual system of house sparrow (Passer domesticus), J. Comp. Physiol. A. 173 (1993), 765-774.
Juss, T.V.S., Davis, I.R., Kollett, B.K. and Mason, R.: Circadian rhythm in neuronal discharge activity in the quail lateral hypothalamic retinoreceipent nucleus (LHRN) recording in vitro, J. Physiol. 475 (1994), 132.
Menaker, M. and Zimmerman, N.: Role of the pineal in the circadian system of birds, Am. Zool. 16 (1976), 45-55.
Gwinner, E.: Effects of pinealectomy on circadian locomotor activity rhythms in European Starling (Sturnus vulgaris), J. Comp. Physiol. A. 126 (1978), 123-129.
Gwinner, E.: Melatonin in the circadian system of birds: model of internal resonance, in T. Hiroshige and K. Homa (eds.), Circadian Clocks and Ecology, Hokkaido Press, Sapporo, 1989, pp. 127-153.
Fulford, G., Forrester, P. and Jones, A.: Modelling with Differential and Difference Equations, Cambridge University Press, Cambridge, 1997.
Arrowsmith, D.K. and Place, C.M.: Ordinary Differential Equations, Chapman & Hall, London, 1982.
Nicolis, G.: Introduction to Nonlinear Science, Cambridge University Press, Cambridge, 1995.
Verhust, F.: Nonlinear Differential Equations and Dynamic Systems (2nd ed.), Springer, Berlin, 1996.
Cassone, V.M. and Brooks, D.S.: Sites of melatonin action in the sparrow brain (Passer domesticus), J. Exp. Zool. 260(3) (1991), 302-309.
Cassone, V.M., Lane, R.F. and Menaker, M.: Melatonin-induced increases in serotonin concentration in specific regions of the chicken brain, Neuroendocrinol. 266 (1986), 171-182.
Cassone, V.M.: Melatonin: time in a bottle', Oxford Review of Reproductive Biology 12 (1990), 319-367.
Takahashi, J.S., Murakami, N., Nikaido, S.S., Pratt, B.L. and Robertson, L.M.: The avian pineal, a vertebrate model system of circadian oscillator: cellular regulation of circadian rhythms by light, second messengers, and macromolecular synthesis, Recent Prog. in Hormone Res. 45 (1989), 279-351.
Halanay, A.: Differential Equations: Stability, Oscillations, Time Lags, Academic Press, New York and London, 1966.
Wiener, J.: Generalized Solutions of Functional Differential Equations, World Scientific, Singapore, 1993.
Diekmann, O., van Gils, S.A., Verduyn Lunel, S.M. and Walther, H.-O.: Delay Equations: Functional-, Complex-, and Nonlinear Analysis, Springer-Verlag, New York, Berlin, 1995.
Gopalsamy, K., Grove, E.A. and Ladas, G.: Neural delay differential equations with variable delays, in: A.R. Aftabizadeh (ed.), Differential Equations and Applications, Ohio University Press, Athens, 1988, pp. 343-347.
French, A.P.: Vibration and Waves, W.W Norton Company. Inc., New York, 1968.
Gaston, S.: The influence of the pineal organ on the circadian activity rhythm in birds, in: M. Menaker (ed.), Biochronmetry, National Academy of Sciences, Washington, D.C., 1971.
Eskin, A.: Some properties of the system controlling the circadian activity of sparrows, in: M. Menaker (ed.), Biochronmetry, National Academy of Sciences, Washington, D.C., 1971.
Pavlidis, T.: Populations of interacting oscillators and circadian rhythms, J. Theor. Biol. 22 (1969), 418-436.
Enright, J.T.: Temporal precision in circadian systems: a reliable neuronal clock from unreliable components, Science 209 (1980), 1542-1545.
Wever, R.A.: Mathematical models of circadian one-and multi-oscillator systems, in: G.A. Carpenter (ed.), Some Mathematical Questions in Biology Circadian Rhythms, The American Mathematical Society, Providence, Rhode Island, 1987, pp. 205-65.
Daan, S. and Beersma, D.: Circadian gating of human-wake cycles, in: M.C. Moore-Ede and C.A. Czeisler (eds.), Mathematical Models of the Circadian Sleep-Wake Cycle, Raven Press, New York, 1984, pp. 129-159.
Winfree, A.T.: The Timing of Biological Clock, Freeman, New York, 1987.
Winfree, A.T.: The Geometry of Biological Time, Springer-Verlag, Berlin, New York, 1990.
Carpender, G.A. and Grossberg, S.: A neural theory of circadian rhythms: the gated pacemaker, Biol. Cybernetics 48 (1983), 35-59.
Forger, D.B., Jewett, M.E. and Kronauer, R.E.: A simpler model of the human circadian pacemaker, Biol. Rhythms 14(6) (1999), 532-537.
Gander, P.H., Kronauer, R.E., Czeisler, C.A. and Moore-Ede, M.C.: Simulating the action of zeitgebers on a coupled two-oscillator model of the human circadian syste, J. Am. Physiol. 247 (1984), R418-R426.
Richie, C.G. and Womack, B.F.: A Mathematical Model For The Biological Clock of Passer Domesticus, Technical Report No. 28, University of Texas, 1966.
Enright, J.T.: Mutual excitation of damped oscillators and self-sustainment of circadian rhythms, in: M.C. Moore-Ede and C.A. Czeisler (eds.), Mathematical Models of the Circadian Sleep-Wake Cycle, Raven Press, New York, 1984, pp. 1-17.
Hoffmann, K.: Splitting of the circadian rhythm as a function of light intensity, in: M. Menaker (ed.), Biochronometry, National Academy of Sciences, 1971, pp. 134-151.
Meijer, J.H., Daan, S., Overkamp, J.F. and Hermann, P.M.: The two-oscillator circadian system of tree shrew (Turpaia belangeri) and its response to light pulse, J. Biol. Rhythms 5 (1990), 1-16.
Pickard, G.E., Turek, F.W. and Sollars, R.J.: Light intensity and splitting in the golden hamster, Physiol. & Behav. 54 (1993), 1-5.
Underwood, H. and Siopes J.T.: Circadian organization in the Japanese quail, J. Exp. Zool. 232 (1984), 557-566.
MacBride, S.E.: Pineal biochemical rhythms of the chicken (Gallus domesticus): light cycles and locomotor activity correlates, Ph.D. Thesis, University of Pittsburgh, Pittsburgh, PA, 1973.
Ebihara, S., Uchiyama, K. and Oshima, I.: Circadian organization in the pigeon, role of the pineal organ and eye, J. Comp. Physiol. 154 (1984), 59-69.
Murakami, N., Takamure, M., Takahashi, K., Utunomiya, K., Kurda, H. and Etoh, T.: Longterm cultured neurons from rat suprachiasmatic nucleus retain the capacity for circadian oscillation of vasopressin release, Brain Res. 545 (1991), 347-350.
Simpson, S.M. and Follett, B.K.: Pineal and hypothalamic pacemakers: their role in regulating circadian rhythmicity in Japanese quail, J. Comp. Physiol. A. 144 (1981), 381-389.
Klein, C.D. and Moore, R.Y.: Pineal N-acetyltransferse and hydroxyindole-Omethyltransferase: control by the retinohypothalamic tract and the suprachiasmatic nucleus, Brain Res. 174 (1979), 245-262.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wu, Hi., Lu, J. & Li, BL. A Coupled Oscillatory Model Mimicking Avian Circadian Regulatory Systems. Journal of Biological Physics 26, 261–272 (2000). https://doi.org/10.1023/A:1010378224387
Issue Date:
DOI: https://doi.org/10.1023/A:1010378224387