Abstract
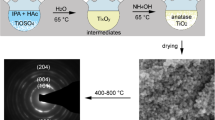
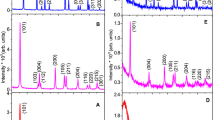
Titania sols, gels and nanopowders have been produced by the controlled hydrolysis of tetraisopropyltitanate (TPT) in sodium bis(2-ethylhexyl)sulfosuccinate (AOT) reverse micelles. Particle formation and aggregation have been investigated by photon correlation spectroscopy, the crystal phases by FT-Raman spectroscopy, and the crystallite dimensions of the precipitates by transmission electron microscopy. Nanoparticles could be produced at relatively high Ti(IV) concentrations (up to 0.05 mol dm−3). These nanoparticles aggregated into sols, with colloid sizes of 20–300 nm, eventually forming gelatinous precipitates. The kinetics of particle formation and aggregation were controlled by varying the primary process parameters [TPT], [H2O]/[AOT] (w0), and [H2O]/[Ti(IV)] (R), yielding a range of products including stable, transparent sols, precipitates and monolithic gels. The aggregation kinetics and physical properties of the sols depended strongly on w0. Different titania phases were produced, depending on w0; w0 ≤ 6 yielded amorphous particles, while w0 ≥ 10 produced anatase. The dimensions of the crystallites were comparable to those of the parent reverse micelles. A model was developed to interpret the effect of the primary process parameters on colloidal stability: (1) nucleation to form primary crystallites occurs by rapid hydrolysis and condensation reactions within the reverse micelle and (2) subsequent colloidal growth by aggregation occurs by reverse micellar exchange, where the rate of growth is governed by electrostatic and steric stability factors which increase as [AOT]/[TPT] (S) and residual [H2O]/[AOT] (wr) increase.
Similar content being viewed by others
References
1._R. Dagani, Chem. Eng. News, November, 18 (1992).
C.J. Brinker and G.W. Scherer, Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing (Academic Press, San Diego, 1990).
P.D.I. Fletcher, A.M. Howe, and B.H. Robinson, J. Chem. Soc., Faraday Trans.1 83, 985 (1987).
C. Guizard, M. Stitou, A. Larbot, L. Cot, and J. Rouviere, in Better Ceramics Through Chemistry III, edited by C.J. Brinker, D.E. Clark, and D.R. Ulrich (Materials Research Society, 1988), p. 115.
K. Osseo-Asare and F.J. Arriagada, in Better Ceramics Through Chemistry III, edited by C.J. Brinker, D.E. Clark, and D.R. Ulrich (Materials Research Society, 1988), p. 3.
W. Wang, X. Fu, J. Tang, and L. Jiang, Colloids Surf. A 81, 177 (1993).
F.J. Arriagada and K. Osseo-Asare, Colloids Surf. A 69, 105 (1992).
K. Osseo-Asare and F.J. Arriagada, Colloids Surf. A 50, 321 (1990).
H. Yamauchi, T. Ishikawa, and S. Kondo, Colloids Surf. A 37, 71 (1989).
C. Guizard, A. Larbot, L. Cot, S. Perez, and J. Rouviere, J. Chim. Phys. Phys.-Chim. Biol. 87, 1901 (1990).
Y. Tricot, R. Rafaeloff, A. Emeren, and J.H. Fendler, ACS Symp. Series 278, 99 (1985).
F.J. Arriagada and K. Osseo-Asare, Refract. Met., Proc. Symp. Annu. Meet. Miner., Met. Mater. Soc. 1991, 259–269 (1991).
T. Hirai, E. Imamura, T. Matsumoto, R. Kuboi, and I. Komasawa, Kagaku Kogaku Ronbunshu 18, 296 (1992).
T. Hirai, H. Sato, and I. Komasawa, Ind. Eng. Chem. Res. 32, 3014 (1993).
J. Livage, M. Henry, and C. Sanchez, Prog. Solid St. Chem. 18, 259 (1989).
P.D. Moran, J.R. Bartlett, J.L. Woolfrey, G.A. Bowmaker, and R.P. Cooney, J. Sol-Gel Sci. Technol. 8, 65 (1997).
A. Maitra, J. Phys. Chem. 88, 5122 (1984).
T.F. Towey, A. Khan-Lodhi, and B.H. Robinson, J. Chem. Soc., Faraday Trans. 86, 3757 (1990).
S. Modes and P. Lianos, J. Phys. Chem. 93, 5854 (1989).
M.P. Pileni, L. Motte, and C. Petit, Chem. Mater. 4, 338 (1992).
L. Motte, C. Petit, L. Boulanger, P. Lixon, and M.P. Pileni, Langmuir 8, 1049 (1992).
C. Petit, P. Lixon, and M.P. Pileni, J. Phys. Chem. 94, 1598 (1990).
P. Lianos and J.K. Thomas, Chem. Phys. Lett. 125, 299 (1986).
P.D.I. Fletcher and B.H. Robinson, Ber. Bunsenges. Phys. Chem. 85, 863 (1981).
S.S. Atik and J.K. Thomas, J. Am. Chem. Soc. 103, 3543 (1981).
M. Smoluchowski, Z. Phys. Chem. 92, 129 (1918).
J. Lang, A. Jada, and A. Malliaris, J. Phys. Chem. 92, 1946 (1988).
R. Jóhannsson, M. Almgren, and J. Alsins, J. Phys. Chem. 95, 3819 (1991).
A.V. Barzykin and M. Tachiya, J. Phys. Chem. 98, 2677 (1994).
D.N.L. McGown, G.D. Parfitt, and E. Willis, J. Colloid Interface Sci. 20, 650 (1965).
C.A. Malbrel and P. Somasundaran, Langmuir 8, 1285 (1992).
K. Kandori, A. Kazama, K. Kon-no, and A. Kitahara, Bull. Chem. Soc. Jpn. 57, 1777 (1984).
S. Tohno and M. Itoh, J. Aerosol Sci. 24, 339 (1993).
J.P. Wilcoxon, R.L. Williamson, and R. Baughman, J. Chem. Phys. 98, 9933 (1993).
E.A. Lissi and D. Engel, Langmuir 8, 452 (1992).
P. Ménassa and C. Sandorfy, Can. J. Chem. 63, 3367 (1985).
P.D.I. Fletcher, M.F. Galal, and B.H. Robinson, J. Chem. Soc., Faraday Trans. I 80, 3307 (1984).
Unpublished results.
G.D. Smith, C.E. Donelan, and R.E. Barden, J. Colloid Interface Sci. 60, 488 (1977).
B. Boddenberg and W. Horstmann, Ber. Bunsenges. Phys. Chem. 92, 519 (1988).
C.J. Brinker and G.W. Scherer, Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing (Academic Press, San Diego, 1990), p. 242.
S. Krishnakumar and P. Somasundaran, Langmuir 10, 2786 (1994).
P.D. Moran, G.A. Bowmaker, R.P. Cooney, J.R. Bartlett, and J.L. Woolfrey, Langmuir 11, 738 (1995), and references therein.
H. Yotsumoto and R.-H. Yoon, J. Colloid Interface Sci. 157, 426 (1993).
B.V. Velamakanni, J.C. Chang, F.F. Lange, and D.S. Pearson, Langmuir 6, 1323 (1990).
J.-L. Look and C.F. Zukoski, J. Am. Ceram. Soc. 78, 21 (1995).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Moran, P.D., Bartlett, J.R., Bowmaker, G.A. et al. Formation of TiO2 Sols, Gels and Nanopowders from Hydrolysis of Ti(OiPr)4 in AOT Reverse Micelles. Journal of Sol-Gel Science and Technology 15, 251–262 (1999). https://doi.org/10.1023/A:1008741109896
Issue Date:
DOI: https://doi.org/10.1023/A:1008741109896