Abstract
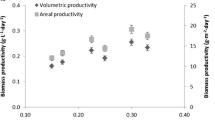
This work concerns an attempt to develop large scalecultivation of Porphyridium sp. outdoors. Theimpact on cell growth and production of solublesulphated polysaccharides of light-path length (LP)was studied in flat plate glass reactors outdoors. TheLP of the plate reactors ranged from 1.3–30 cm,corresponding to culture volumes of 3–72 L. The sidewalls of all reactors were covered, ensuring similarilluminated surfaces for all reactors. Maximal daytemperature was maintained at 26 ±1 °C.Growth conditions of pH (7.5), stirring (withcompressed air) and mineral nutrients, were optimal.Maximal volumetric concentration of the soluble sulfated polysaccharide (1.32 g L-1) was obtained in winter with the smallest light-pathreactor (1.3 cm ) at a cell density of 1.37 ×1011cells L-1. Under these conditions, theviscosity of the culture medium was also highest,being inversely proportional to the culture'slight-path. Highest areal concentration of solublepolysaccharides (60 g m-2) and areal cell density(3.01 × 1012m-2) was recorded in the 20 cmLP reactor, progressively lower values being obtainedas the light path became shorter. A similar patternwas obtained for the areal productivity ofpolysaccharides, the highest being 4.15 g m-2day-1 (considering the total illuminated reactorsurface), produced in the 20-cm LP reactor.The main sugar composition (i.e. xylose, galactose andglucose) of the sulfated polysaccharides was similarin all reactors. As viscosity increased with timeduring culture growth, there was a substantial declinein bacterial population. Cultivation throughout mostof the year provided good evidence that a light pathlength of 20 cm in flat plate reactors under theseconditions is optimal for maximal areal solublepolysaccharide production of Porphyridium sp.
Similar content being viewed by others
References
Albersheim P, Nevin DJ, English PD, Karr A (1967) A method for analysis of sugars in plant cell wall polysaccharides by gas liquid chromatography. Carbohyd. Res. 5: 340-345.
Arad (Malis) S (1999) Polysaccharides of red microalgae. In Cohen Z (ed.) Chemicals from Microalgae. Taylor and Francis, London, pp. 282-291.
Chaumont D, Thepenier C, Gudin C (1988) Scaling up a tubular photoreactor for continuous culture of Porphyridium cruentum from laboratory to pilot plant (1981-1987). In Stadler T, Mollion J, Verdus M-C, Karamanos Y, Morvan H, Christiaen D (eds), Algal Biotechnology. Elsevier Applied Science, London, pp. 199-208.
Cohen Z, Vonshak A, Boussiba S, Richmond A (1988) The effect of temperature and cell concentration on the fatty acid composition of outdoor cultures of Porphyridium cruentum. In Stadler T, Mollion J, Verdus M-C, Karamanos Y, Morvan H, Christiaen D (eds.), Algal Biotechnology. Elsevier Applied Science, London, pp. 421-430.
Cohen E, Arad S (1989) A closed system for outdoor cultivation of Porphyridium. Biomass 18: 59-67.
Dermoun D, Chaumont D, Thebault J, Dauta A (1992) Modeling of growth of Porphyridium cruentum in connection with two interdependent factors: light and temperature. Biores. Technol. 42: 113-117.
Dubois M, Gilles KA, Hamilton JK, Rebers PA Smith F (1956) Colorimetric method for determination of sugar and related substances. Anal. Chem. 28: 350-356.
Geresh S, Arad (Malis) S (1991) The extracellular polysaccharides of the red microalgae: chemistry and rheology. Biores. Technol. 38: 195-201.
Gudin C, Chaumont D (1991) Cell fragility-the key problem of micro-algae mass production in closed photobioreactors. Biores. Technol. 38: 145-155.
Hu Q, Guterman H, Richmond A (1996) A flat inclined modular photobioreactor for outdoor mass cultivation of photoautotrophs. Biotechnol. Bioengng 51: 51-60.
Hu Q, Richmond A (1996) Productivity and photosynthetic efficiency of Spirulina platensis as affected by light intensity, cell density and rate of mixing in a flat plate photobioreactors. J. appl. Phycol. 8: 139-145.
Hu Q, Zarmi Y, Richmond A (1998) Combined effects of light intensity, light-path and culture density on output rate of Spirulina platensis (Cyanobacteria). Eur. J. Phycol. 33: 165-171.
Jones RE, Speer HL, Kury W (1963) Studies on the growth of red alga Porphyridium cruentum. Physiol. Plant. 16: 636-643.
Lee YK, Tan HM (1988) Effect of temperature, light intensity and dilution rate on the cellular composition of red alga Porphyridium cruentum in light limited chemostat cultures. MIRCEN J. 4: 231-237.
Ramus J (1972) The production of extracellular polysaccharide by the unicellular red alga Porphyridium aerugineum. J. Phycol. 8: 97-111.
Richmond A (1999) Physiological principles and modes of cultivation in mass production of photoautotrophic microalgae. In Cohen Z (ed.) Chemicals from Microalgae. Taylor and Francis, London, pp. 353-386.
Sommerfeld MR, Nichols HW (1970) Comparative studies on the genus Porphyridium Naeg. J. Phycol. 6: 67-78.
Vonshak A, Cohen Z, Richmond A (1985) The feasibility of mass cultivation of Porphyridium cruentum. Biomass 8: 13-25.
Zou N, Richmond A (1999) Effect of light-path length in outdoor flat plate reactors on output rate of cell mass and of EPA in Nannochloropsis sp. J. Biotechnol. 70: 351-356.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Singh, S., Arad, S.(. & Richmond, A. Extracellular polysaccharide production in outdoor mass cultures of Porphyridium sp. in flat plate glass reactors. Journal of Applied Phycology 12, 269–275 (2000). https://doi.org/10.1023/A:1008177002226
Issue Date:
DOI: https://doi.org/10.1023/A:1008177002226