Abstract
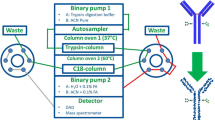
The monoclonal-antibody production of an immobilized hybridoma cell line cultivated in a fluidized-bed reactor was monitored on-line for nearly 900 h. The monoclonal antibody concentration was determined by an immuno affinity-chromatography method (ABICAP). Antibodies directed against the product, e.g. IgG, were immobilized on a micro-porous gel and packed in small columns. After all IgG present in the sample was bound to the immobilized antibodies, unbound proteins were removed by rinsing the column. Elution of the bound antibodies followed and the antibodies were determined by fluorescence. The analytical procedure was automated with a robotic device to enable on-line measurements. The correlation between the on-line determined data and antibody concentrations measured by HPLC was linear.
A sampling system was constructed, which was based on a pneumatically actuated in-line membrane valve integrated into the circulation loop of the reactor. Separation of the cells from the sample stream was achieved by a depth filter made of glass-fibre, situated outside the reactor. Rapid obstruction of the filter by cells or cell debris and contamination of the sample system was avoided by intermittent rinsing of the sample system with a chemical solution. The intermittent rinsing of the filter, which had a surface of 4.8 cm2, resulted in an operational capacity of up to 40 samples (1.0 l total sample volume). Both the sampling system and the analytical device functioned without failure during this long-term culture.
The culture temperature was varied between 34 and 40 °C. Raising the temperature from 34 up to 37 °C resulted in a simultaneous increase of growth and specific antibody production rate. Specific metabolic rates of glucose, lactate, glutamine and ammonium stayed constant in this temperature range. A further enhancement of temperature up to 40 °C had a negative effect on the growth rate, whereas the specific monoclonal antibody production rate showed a small increase. The other specific metabolic rates also increased in the temperature range between 38 to 40 °C.
Similar content being viewed by others
References
Barnabé N and Butler M (1994) Effect of temperature on nucleotide pools and monoclonal antibody production in a mouse hybridoma. Biotechnol. Bioeng. 44: 1235–1245.
Bergmeyer HU, Bergmeyer J and Graßl M (eds.) (1985) Methods of enzymatic analysis, 3th edition, 8, (pp. 454–461) VCH, Weinheim.
Birnbaum S, Bülow L, Hardy K, Danielsson B and Mosbach K (1986) Automated thermometric enzyme immunoassay of human proinsulin produced by Eschericia coli. Anal. Biochem. 158: 12–19.
Bloemkolk J, Gray MR, Merchant F and Mosmann TR (1992) Effect of temperature on hybridoma cell cycle and mab production. Biotechnol. Bioeng 40: 427–431.
Degelau A, Freitag R, Linz F, Middendorf C, Scheper T, Bley T, Müller S, Stoll P and Reardon KF (1992) Immuno-and flow cytometric analytical methods for biotechnological research and process monitoring. J. Biotechnol. 25: 115–144.
Freitag R, Scheper T and Schügerl K (1991) Development of a turbimetric immunoassay for on-line monitoring of proteins in cultivation processes. Enzyme Microb. Technol. 13: 969–975.
Gebbert A, Alvarez-Icaza M, Peters H, Jäger V, Bilitewski U and Schmidt RD (1994) On-line monitoring of monoclonal antibody production with regenerable flow injection immuno systems. J. Biotechnol. 31: 213–220.
Goergen JL, Marc A and Engasser JM (1993) Determination of cell lysis and death kinetics in continuous hybridoma cultures from measurements of lactate dehydrogenase release. Cytotechnology 11: 189–195.
Hartmann H, Lübbers B, Casaretto M, Bautsch W, Klos A and Kohl J (1993) Rapid quantification of C3a and C5a using a combination of chromatographic and immunoassay procedures. J. Immunol. Methods 166: 35–44.
Jo E, Park H, Kim D and Moon HM (1993) Repeated fed-batch culture of hybridoma cells in nutrient-fortified high-density medium. Biotechnol. Bioeng. 42: 1229–1237.
Lüllau E, Dreisbach C, Grogg A, Biselli M and Wandrey C (1992) Immobilization of animal cells on chemically modified siran carrier. In: Spier RE, Griffiths JB and MacDonalds C (eds.) Animal Cell Culture Technology: Developments, Processes and Products (pp. 469–474) Butterworth-Heinemann, Oxford.
Mattiasson B, Berdén P and Ling TGI (1989) Flow injection binding assays: a way to increase the speed in binding assays. Anal. Biochem. 181: 379–382.
Mattiasson B and Hakanson H (1992) Immunochemically based assays for process control. In: Fiechter A (ed.) Advances in biochemical engineering/biotechnology. Vol. 46 (pp. 81–102) Springer-Verlag, Berlin.
Murthy U, Basu A, Rodeck U, Herlyn M, Ross AH and Das M (1987) Binding of an antagonistic monoclonal antibody to an intact and fragmented EGF-receptor polypeptide. Arch. Biochem. Biophys. 252: 549–560.
Nilsson M, Mattiasson G and Mattiasson B (1993) Automated immunochemical binding assay (flow-ELISA) based on repeated use of an antibody column placed in a flow-injection system. J. Biotechnol. 31: 381–394.
Philips HJ (1973) Dye exclusion tests for cell viability. In: Kruse PF and Patterson MK (eds.) Tissue culture (pp. 406–408) Academic Press, New York.
Ramirez OT and Mutharasan R (1990) The role of the plasma membrane fluidity on the shear sensitivity of hybridomas grown under hydrodynamic stress. Biotechnol. Bioeng. 36: 911–920.
Robinson DK, Chan CP, Yu Ip C, Tsai PK, Tung J, Seamans TC, Lenny AB, Lee DK, Irwin J and Silberklang M (1994) Characterization of a recombinant antibody produced in the course of a high yield fed-batch process. Biotechnol. Bioeng. 44: 727–735.
Scheper T (1991) Bioanalytik: Messung des Zellzustand und der Zellumgebung in Bioreaktoren. Vieweg, Braunschweig.
Schügerl K (1993) Which requirements do flow injection analyzer/biosensor systems have to meet for controlling the bioprocess? J. Biotechnol. 31: 241–256.
Stamm WW, Pommerenning G, Wandrey C and Kula M (1989) On-line measurement of extracellular proteins in the continuous cellulase production by flow injection analysis (FIA). Enzyme Microb. Technol. 11: 96–105.
Stöcklein W and Schmid RD (1990) Flow-injection immunoanalysis for the on-line monitoring of monoclonal antibodies. Anal. Chim. Acta 234: 83–88.
Sureshkumar GK and Mutharasan R (1990) The influence of temperature on a mouse-mouse hybridoma growth and monoclonal antibody production. Biotechnol. Bioeng. 37: 292–295.
Thömmes J, Gätgens J, Biselli M, Rundstadler PW and Wandrey C (1993) The influence of dissolved oxygen tension on the metabolic activity of an immobilized hybridoma population. Cytotechnology 13: 29–39.
Van der Pol JJ, Spohn U, Eberhardt R, Gätgens J, Biselli M, Wandrey C and Tramper J (1994) On-line monitoring of an animal cell culture with multi-channel flow injection analysis. J. Biotechnol. 37: 253–264.
Vassualt A (1974) Lactate dehydrogenase: UV-method with pyruvate and NADH. In: Bergmeyer HU (ed.) Methods of enzymatic analysis. Vol 3 (pp. 118–138) Academic Press, New York.
Wagner A, Marc A, Engasser JM and Einsele A (1992) The use of lactate dehydrogenase (LDH) release kinetics for the evaluation of death and growth of mammalian cells in perfused reactors. Biotechnol. Bioeng. 39: 320–326.
Werner RG, Walz F, Noé W and Konrad A (1992) Safety and economic aspects of continuous mammalian cell culture. J. Biotechnol. 22: 51–68.
Worsfold PJ, Hughes A and Mowthorpe DJ (1985) Determination of human serum immunoglobulin G using Flow Injection Analysis with rate turbimetric detection. Analyst 110: 1303–1305.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
van der Pol, J.J., Machnik, M., Biselli, M. et al. On-line immunoanalysis of monoclonal antibodies during a continuous culture of hybridoma cells. Cytotechnology 24, 19–30 (1997). https://doi.org/10.1023/A:1007913128209
Issue Date:
DOI: https://doi.org/10.1023/A:1007913128209