Abstract
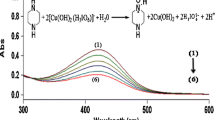
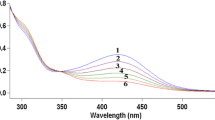
The nature of the diperiodatocuprate(III) (DPC) species present in aqueous alkaline medium has been investigated by a kinetic and mechanistic study on the oxidation of iodide by DPC. The reaction kinetics were studied over the 1.0 × 10−3–0.1 mol dm−3 alkali range. The reaction order with respect to DPC, as well as iodide, was found to be unity when [DPC] ≪ [I−]. In the 1.0 × 10−3–1.0 × 10−2 mol dm−3 alkali region, the rate decreased with increase in the alkali concentration and a plot of the pseudo-first order rate constant, k versus 1/[OH−] was linear. Above 5.0 × 10−2 mol dm−3, a plot of k versus [OH−] was also linear with a non-zero intercept. An increase in ionic strength of the reaction mixtures showed no effect on k at low alkali concentrations, whereas at high concentrations an increase in ionic strength leads to an increase in k. A plot of 1/k versus [periodate] was linear with an intercept in both alkali ranges. Iodine was found to accelerate the reaction at the three different alkali concentrations employed. The observed results indicated the following equilibria for DPC.
[Cu(H2IO6)2]3- ⇋ [Cu(H2IO6)]- + H2IO6 3-
[Cu(H2IO6)] + OH- ⇋ [Cu(HIO6)]- + H2O
A suitable mechanism has been proposed on the basis of these equilibria to account for the kinetic results.
Similar content being viewed by others
References
L. Malatesta, Gazz. Chim. Ital., 71, 467 (1941).
W. Levason and M.D. Spicer, Coord. Chem. Rev., 75, 45 (1987).
A. Balikungeri, M. Pelletier and D. Monnier, Inorg. Chim. Acta, 22, 7 (1977); ibid., 29, 141 (1978).
M.W. Lister, Candian J. Chem., 31, 638 (1953); ibid., 39, 2330 (1961).
G.I. Rozovskii, Tovarnye. Zn. Alc. (1971); Chem. Abstr., 74, 55808c (1971).
M.G. Ram Reddy, B. Sethuram, and T. Navneeth Rao, Indian J. Chem., 16A, 31 (1978).
C.P. Murthy, B. Sethuram and T. Navneeth Rao, Z. Phys. Chem. (Leipzig) (1981); Chem. Abstr., 94, 98199d (1981); ibid. (1989), Chem. Abstr., 111, 220085g (1989).
K. Bal Reddy, B. Sethuram and T. Navneeth Rao, Indian J. Chem., 20A, 395 (1981); ibid., 27A, 730 (1988).
B. Sethuram, S. Padmaja, K. Nageswar and T. Navaneeth Rao, Indian J. Chem., 32A, 685 (1993).
Shan Jinhuan Liu and Tieying Wuli, Huaxue Xuebao, 10, 947 (1994); Chem. Abstr., 122, 55453t (1995).
V.J. Shiner and C.R. Wasmuth, J. Am. Chem. Soc., 81, 37 (1959).
J. Aveston, J. Chem. Soc., 273 (1969).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nadimpalli, S., Padmavathy, J. & Yusuff, K.K.M. Determination of the nature of the diperiodatocuprate(III) species in aqueous alkaline medium through a kinetic and mechanistic study on the oxidation of iodide ion. Transition Metal Chemistry 26, 315–321 (2001). https://doi.org/10.1023/A:1007116932047
Issue Date:
DOI: https://doi.org/10.1023/A:1007116932047