Abstract
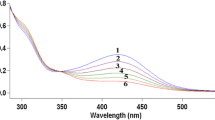
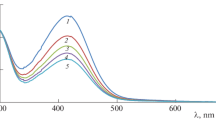
The kinetics of oxidation of piperazine by the copper complex, diperiodatocuprate(III) in alkaline medium was studied at 298 K, at an ionic strength of 2.0 x 10 −2 mol dm −3. The reaction between piperazine and diperiodatocuprate(III) in aqueous alkaline medium exhibited 1:2 stoichiometry. The oxidation products were identified by UV-Visible, GC-MS and IR spectral studies. In the present study we have obtained different kinetic observations. The reaction exhibited unit order in case of diperiodatocuprate(III), while less than unit order with respect to piperazine. The addition of alkali and periodate retarded the rate of reaction. The effects of added products, ionic strength and dielectric constant on the rate of the reaction were also studied. The active species of diperiodatocuprate(III) in alkaline media is [Cu(OH) 2(H 3 IO 6)] −. The activation parameters with respect to the rate determining step and the thermodynamic quantities with respect to the equilibrium steps were evaluated and discussed. The plausible mechanism consistent with the experimental results was proposed and discussed in detail.

The kinetics of oxidation of Piperazine by Diperiodatocuprate(III) complex in aqueous alkaline medium was studied spectrophotometrically. The possible mechanism was proposed based on the experimental rate laws. The reaction constants involved in the different steps of mechanism were evaluated and thermodynamic quantities were also calculated and discussed.







Similar content being viewed by others
References
Reddy B, Sethuram B and Navaneeth Rao T 1984 Indian J. Chem 23A 593
Levason W and Spicer M D 1987 Coord. Chem. Rev 76 45
Balikungeri A, Pelletier M and Monnier D 1977 Inorg. Chim. Acta 22 7
Niu W, Zhu Y, Hu K, Tong C and Yang H 1996 Int. J. Chem. Kinet 28 899
Rozovoskii G I, Misyavichyus A K and Prokopchik A Y 1975 Kinet. Catal. 16 337
Kitajima K N and Moro-oka Y 1994 Chem. Rev. 94 737
Ramreddy M G, Sethuram B and Navaneeth Rao T 1978 Indian J. Chem 16A 313
Kovat Z 1960 Acta. Chim. Hung 22 313
Crenshaw G L and Shaver R J 1956 In Down to Earth (Michigan: Dow Chemical Company)
Maynard W R Jr. 1961 J. AOAC Int. 42 610
Goodman L S and Gilman A 1956 In The Pharmacological Basis of Therapeutics, 2nd Edition (New York: Macmillan)
Jouve A, Grass A and Benyamine R 1963 Vie Med. 44 115
Mc Nair T J, Wibin F A, Hoppe E T, Schmidt J L and de Peyster F A 1963 J. Surg. Res. 3 130
Mikhalev V A, Dorokhova M I and Smolina N E 1963 Meditsinskaia promyshlennost’ SSSR 17 17
Foye W O and Kay D H 1962 J. Pharm. Sci. 51 1098
Kiran T S, Hiremath D C and Nandibewoor S T 2007 Z. Phys. Chem. 221 501
Shetti N P, Hegde R N and Nandibewoor S T 2009 Cent. Eur. J. Chem. 7 929
Aravindakshan K K and Muraleedharan K 1990 Thermochim. Acta 159 101
Chandrashekar, Venkatesha B M, Ananda S and Made Gowda N M 2013 Modern Research in Catalysis 2 157
Panigrahi G P and Misro P K 1978 Ind. J. Chem. 16A 201
Jaiswal P K and Yadava K L 1973 Ind. J. Chem. 11 83
Murthy C P, Sethuram B and Navaneeth Rao T 1981 Z. Phys. Chem. 262 336
Jeffery G H, Bassett J, Mendham J and Denny R C 1996 In Vogel’s Textbook of Quantitative Chemical Analysis 5th ed. (Essex UK: ELBS, Longman) p. 455
(a) Kolthoff I M, Meehan E J and Carr E M 1953 J. Am. Chem. Soc. 75 1439; (b) Hiremath G C, Mulla R M and Nandibewoor S T 2005 J. Chem. Res. 197
Kiran T S, Hiremath C V and Nandibewoor S T 2006 Appl. Cat. A: Gen. 305 79
Sethuram B 2003 In Some aspects of electron transfer reactions involving organic molecules (New Delhi: Allied Publishers) p. 78
Lister M W 1953 Can. J. Chem. 31 638
Hegde R N, Shetti N P and Nandibewoor S T 2009 Polyhedron 28 3499
Hosamani R R, Hedge R N and Nandibewoor S T 2010 Monatsh. Chem. 141 1069
Cohen G L and Atkinson G 1964 Inorg. Chem. 3 1741
Pauling L 1969 In The Nature of the Chemical Bond 3rd ed. (New Delhi: Oxford & IBH publishers)
Walling C 1957 In Free Radicals in Solution (New York: Academic Press)
Farokhi S A and Nandibewoor S T 2003 Tetrahedron 59 7595
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
The order with respect to alkali concentration and periodate concentration were obtained from the plot of log k obs versus log [OH −] (figure S1) and log k obsversus log\([\text {IO}_{4}^{-}]\) (figure S2). Supplementary Information is available at www.ias.ac.in/chemsci.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
[DPC] f and [DPC] T are given as,
where, C denotes {DPC-PPZ} complex.
Under the experimental conditions, [OH −] f∼ 10 3[DPC] T and [PPZ] T∼(2–10)[DPC] T (see table 1). Hence, corrections for [OH −] f and [PPZ] f due to interaction with DPC were not applied (i. e., [KOH] added= [OH −] f and [PPZ] f= [PPZ] T were assumed).
Substituting equation (A5) in equation (A1), we get,
Rights and permissions
About this article
Cite this article
PATTAR, V.P., MAGDUM, P.A., PATIL, D.G. et al. Thermodynamic, kinetic and mechanistic investigations of Piperazine oxidation by Diperiodatocuprate(III) complex in aqueous alkaline medium. J Chem Sci 128, 477–485 (2016). https://doi.org/10.1007/s12039-016-1044-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-016-1044-x