Abstract
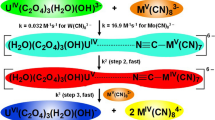
The kinetics and mechanism of the reduction of diaquotetrakis(2,2′-bipyridine)-µ-oxodiruthenium(III), [(H2O)2(bipy)4Ru2O]4+, by H3PO2 has been studied in aqueous acid at ionic strength = 0.5 mol dm−3 (NaClO4), [H+] = 5.0 × 10−2 mol dm−3 and temperature = 31 ± 1 °C. Measurement of the stoichiometry showed that 1 mole of [(H2O)2(bipy)4Ru2O]4+ was reduced by 1 mole of H3PO2. The reaction was found to be first order with respect to both [(H2O)2(bipy)4Ru2O4+] and [H3PO2], hence second order overall. Variations in the ionic strength and dielectric constant of the reaction medium had no effect on the rate. Also, addition of various ions to the reaction medium did not significantly alter the rate. Free radicals were identified during the course of the reaction by a polymerisation test. Spectroscopic information and Michaelis–Menten plots suggested the absence of an intermediate complex prior to electron transfer. [(H2O)2(bipy)2Ru]2+, the reduction product of [(H2O)2(bipy)4Ru2O]4+, plus H3PO3, the oxidation product of H3PO2, were identified in the product solutions. It is suggested that the reaction proceeds through the outer sphere pathway. A mechanism for the reaction is proposed.




Similar content being viewed by others
References
Weaver TR, Meyer TJ, Adeyemi SA, Brown GM, Ecberg RP, Hatfield WE, Johnson EC, Murray RW, Untereker D (1975) Chemically significant interactions between ruthenium ions in oxo-bridged complexes of ruthenium(III). J Am Chem Soc 97:3039–3047
Griffiths WP (1970) Transition Metal Oxo-Complexes. Coord Chem Rev 5:459–517
Meyer TJ, Huynh MHV (2003) The remarkable reactivity of high oxidation state of ruthenium and osmium polypyridyl complexes. Inorg Chem 42:8140–8160
Gersten SW, Samuels GJ, Meyer TJ (1982) Catalytic oxidation of water by an oxobridged ruthenium dimer. J Am Chem Soc 104:4029–4030
Gilbert JA, Eggleston DS, Murphy WR, Geselowitz DA, Gersten SW, Hodgson DJ, Meyer TJ (1985) Structure and redox properties of the water-oxidation catalyst [(bipy)2(OH2)RuORu(OH2)(bipy)2]4+. J Am Chem Soc 107:3855–3866
Meyer TJ (1990) Intramolecular control of excited state electron and energy electron transfer. Pure Appl Chem 62:10–16
Balzani V, Juris A, Ventura M, Campagna S, Serroni S (1996) Luminescent and redox-active polynuclear transition metal complexes. Chem Rev 96:759
Kalyanasundaram K, Gratzel M (1998) Applications of functionalised transition metal complexes in photonic and optoelectronic devices. Coord Chem Rev 177:347–414
Juris A, Balzani V, Barigelleti F, Campagna S, Belser P, Vonzelewsky A (1998) Ru(II) Polypyridine Complexes: Photophysics, Photochemistry, Electrochemistry and Chemiluminescence. Coord Chem Rev 84:85–89
Hammerstrom L, Sun LC, Akermark B, Styring S (2000) Mimicking photosystem ii reactions in artificial photosynthesis. Catal Today 58:51–59
Islam A, Sughara H, Arakawa H (2003) Molecular design of ruthenium(ii) polypyridyl photosensitizers for efficient nanocrystalline TiO2 solar cells. J Photochem Photobiol A 158:131–138
Barigelleti F, Flamigni L (2000) Photoactive molecular wires based on metal complexes. Chem Soc Rev 29:1–14
El-Ghayoury A, Harriman A, Khaytr A, Ziesel R (2000) Controlling electronic communication in ethynylated-polypyridine metal complexes. Ang Chem Int Edit Engl 39:185–188
Mishra L, Yadaw AK, Govil G (2003) Tailored ruthenium polypyridyl complexes as molecular electronic materials. Indian J Chem Sect A 42:1797–1814
Newkome GR, Cho TJ, Moorefield CN, Mohapatra PP, Grodinez LA (2004) Towards Ordered Architectures: Self-Assembly and Stepwise Procedures to the Hexameric Metallomacrocytes [Arylbis(Terpyridinyl)6 FeIINRuII−N]. (N=0, 2, 3, 5). Eur J Chem Sect A 10:1493–1500
Iyun JF, Ayoko GA, Lawal HM (1992) The Stoichiometry and Kinetics of Oxidation of 1,4-Benzenediol by Diaquotetrakis(2,2′-Bipyridine)-µ-oxodiruthenium(III) Cation in Perchloric Media. Indian J Chem Sect A 31:943–947
Iyun JF, Ayoko GA, Lohdip YN (1992) The kinetics and mechanism of the oxidation of diaquotetrakis(2,2′-bipyridine)-µ-oxodiruthenium(III) by bromate in aqueous perchloric acid. Polyhedron 11(18):2277–2433
Iyun JF, Ayoko GA, Lawal HM (1992) Kinetics and mechanism of the oxidation of iodide by diaquotetrakis(2,2I–bipyridine)-µ-oxodiruthenium(III) ion in acid medium. Transit Met Chem 17(1):63–65
Iyun JF, Ayoko GA, Lohdip YN (1992) The oxidation of sulphite by diaquotetrakis(2,2′-bipyridine)-µ-oxodiruthenium(III) ion in perchloric acid. Bull Chem Soc Ethiopia 6(1):1–9
Iyun JF, Ayoko GA, Lawal HM (1995) The kinetics and mechanism of the reduction of diaquotetrakis(2, 2′-bipyridine)-µ-oxodiruthenium(III) by ascorbic acid. Transit Met Chem 20(1):30–33
Iyun JF, Musa KY, Ayoko GA (1995) Oxidation of 2- mercaptoethanol and 2-mercaptoethylamine by [(bpy)2H2O]RuIII]2O4+ in Aqueous Media. Indian J Chem Sect A 34:635–638
Iyun JF, Ayoko GA, Lawal HM (1996) Kinetics of the reduction of µ-oxobis [aquobis(2, 2′- bipyridine)] ruthenium(III) by l–cysteine in aqueous solution. Indian J Chem Sect A 35:210–213
Mohammed Y, Idris SO, Iyun JF (2014) Redox kinetics and mechanism of the oxidation of thiourea by diaquotetrakis (2,2′-bipyridine)-µ-oxodiruthenium(III) ions in aqueous perchloric acid. Int Res J Pure Appl Chem 4(6):819–833
Mohammed Y, Idris SO, Iyun JF (2014) Investigations into the kinetics and mechanism of the electron transfer reactions of n-methylthiourea and diaquotetrakis (2,2′-bipyridine)-µ-oxodiruthenium(III) ions in aqueous acidic medium. Int Res J Nat Appl Scie 1(7):13
Mohammed Y, Idris SO, Iyun JF (2015) Electron transfer reactions of thiosulfate ions and diaquotetrakis (2,2′-bipyridine)-µ-oxodiruthenium(III) ions in aqueous perchloric acid. J Appl Chem Sci Int 2(2):75
Mohammed Y, Idris SO, Onu AD, Iyun JF (2016) Studies of the kinetics and mechanism of the electron transfer reactions of diaquotetrakis (2,2′-bipyridine)-µ-oxodiruthenium(III) ions and dithionite in aqueous medium. FUW Trends Sci Tech J 1(1):11
Carrol RL, Thomas LB (1966) Kinetics and mechanism of oxidation of hypophosphorous acid by cerium (iv) in perchloric acid solution. J Am Chem Soc 88(7):1376–1381
Mishra SK, Gupta YK (1967) Kinetics and mechanism of oxidation of hypophosphite by ce (iv) in sulphuric acid solution. J Inorg Nucl Chem 29:1643–1656
Gupta KS, Gupta YK (1970) Kinetics and mechanism of the reduction of thallium(III) by hypophosphite. J Chem Soc A 256–262
Sengupta KK, Chakladar JK, Chatterjee AK (1973) Kinetics and mechanisms of the oxidation of hypophosphorous and phosphorous acids by chromium(IV). J Inorg Nucl Chem 35(3):901–908
Indrayan AK, Mishra SK, Gupta Y (1981) Kinetics and mechanism of oxidation of hypophosphorous acid with Ag(II) in aqueous perchloric acid solution. Inorg Chem 20(2):450–454
Vogel AI (1978) Vogels’s textbook of quantitative inorganic analysis: including elementary instrumental analysis. ELBS and Longman, London, pp 125–247
Iyun JF, Adegite A (1990) Kinetics and mechanism of the reduction of dichlorotetrakis(2,2′-bipyridine)-µ-oxodiruthenium ion by Ti(III)-EDTA in aqueous acidic medium. Bull Chem Soc Ethiopia 4:27–31
Vaidya VK, Pitlia RL, Kabra BV, Mali SL, Ameta SC (1991) Dye-sensitized photo-oxidation of thiourea by singlet oxygen. J Photochem Photobiol Sect A 60(1):47–50
Ukoha PO (1999) Kinetics and mechanisms of some redox reactions of - µ-oxo-bridged iron(III) complex ion [(Fehedta)2O]2- and some oxyanions and thiols. Ph.D Thesis, Ahmadu Bello University, Zaria, Nigeria and The References therein
Ukoha PO, Iyun JF (2001) Kinetics of reduction of an iron (III) complex ion by mercaptoethanol and mercaptoethylamine in perchloric acid medium. J Chem Soc Nig 26(2):163–168
Ukoha PO, Iyun JF (2002) Oxidation of l-ascorbic Acid by enH2[(FeHEDTA)2O]. 6H2O in aqueous medium. J Chem Soc Nig 27(2):119–122
Ukoha PO, Ibrahim E (2004) Mechanism of the oxidation of β–mercaptoacetic acid by trioxoiodate(V) in aqueous acid medium. Chemclass J 138–141
Zaidi SAH (1991) The Influence of Dielectric Constant Variations on the Kinetics of Reaction Between Bromate and Tellurite Ions in Aqueous Ethanol Mixed Solvents. J Chem Soc Pak 13(2):67–70
Davies NR, Mullins TL (1967) Substitution reactions of some bis (2,2′-bipyridine) and mixed 2,2′-bipyridine, 2,2′,2′-terpyridine complexes of ruthenium (II). Austr J Chem 20(4):657–658
Yusuf UF, Iyun JF, Ayoko GA (2004) Oxidation of hypophosphorous acid by poly(pyridine)iron(III) complexes. ChemClass J 118–122
Dubey S, Hemkar S, Khandelwal CL, Sharma PD (2002) Kinetics and mechanisms of the oxidation of hypophosphorous acid by peroxomonosulphate in aqueous acid medium. Inorg Chem Commun 5(10):903–908
Sengupta KK, Basu B, Sengupta S, Nandi S (1983) Kinetics of the oxidation of hypophosphite ion by Au(III) in hydrochloric acid medium. Polyhedron 2(10):983–986
Zahonyi-Budo E, Simandi LT (1991) Oxidation of hypophosphorous acid by permanganate ion. Inorg Chim Acta 181(2): 149–151
Hightower SM, Lorenz BB, Bernard JG, Johnson MD (2012) Oxidation of phosphorus centres by ferrate(VI): spectral observation of an intermediate. Inorg Chem 51(12):6626–6632
Adamson AW, Gonic E (1963) Reaction of cobalt(II) ethylenediamminetetraacetate with ferricyanide. Inorg Chem 2:129
Huchital DH, Wilkins RG (1967) A study of the intermediates formed in the reaction of ethylenediamminetetraacetatocobaltate(II) with ferricyanide ion. Inorg Chem 6(5):1022–1025
Benetti HP, Caldin EF (1971) Kinetics of solvent and ligand subsitution reactions of metal ions in relation to structural properties of the solvent. J Am Chem Soc A 2198–2207
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mohammed, Y., Idris, S.O. & Onu, A.D. Kinetics and mechanism of the reduction of diaquotetrakis (2,2′-bipyridine)-µ-oxodiruthenium(III) ion by hypophosphorous acid in acidic medium. Transit Met Chem 42, 323–329 (2017). https://doi.org/10.1007/s11243-017-0135-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-017-0135-y