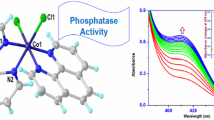
Abstract
The hydrolysis kinetics of p-nitrophenyl acetate (NA) catalyzed by CuII, ZnII and CoII complexes of tris(2-benzimidazylmethyl)amine (NBT) have been studied. The hydrolysis rate is first-order in both metal(II) complex and NA. The second-order rate constants, kcat are 0.083, 0.241 and 0.285 mol−1Ls−1 (298 K, I = 0.10 molL−1 KNO3, 0.02 molL−1 tris buffer, 40% MeCN aqueous solution) for Zn–NBT, Co–NBT and Cu–NBT complexes, respectively. The result indicates that the hydrolytic metalloenzyme activity of different metal complexes increases with the electrophilicity of the metal ions and that the complexes, in this paper, constitute that most efficient hydrolytic metalloenzyme models reported to date. An increase in MeCN content in the solution greatly reduces the hydrolytic activity of the nucleophiles.
Similar content being viewed by others
References
C.I. Branden, H. Jornvall, H. Eklund and B. Furugren in P.D. Boyer (ed.), The Enzymes, 3rd ed., Academic Press, New York, 1975, Vol. 11, pp. 104-190.
A.R. Shatzman and D.J. Kosman, Arch. Biochem. Biophys., 194, 226 (1979).
J.F. Richardson, K.A. Thomas, B.H. Rubin and D.C. Richardson, Proc. Natl. Acad. Sci. U.S.A., 72, 134 (1975).
F. Botre, G. Gros and B.T. Storey (eds) Carbonic Anhydrase, VCH, New York, 1990.
H. Eklund, A.A. Jones and G. Schneider, I. Bertini, C. Luchinat and W. Maret (eds), Zinc Enzymes, Birkhauser, Boston, 1986, 377-392.
A. Neuberger and K. Brocklehurst (eds), Hydrolytic Enzymes, Elsevier Science, Amsterdam 1987.
M.J. Young, D. Wahnon, R.C. Hynes and J. Chin, J. Am. Chem. Soc., 117, 9441 (1995).
(a) J. Chin and M. Banaszczyk, J. Am. Chem. Soc., 111, 2724 (1989); (b) A.M. Sargeson, J.M. Harrow®eld and V. Norris, ibid, 98, 7282 (1976); (c) J.T. Groves and L.A. Baron, ibid, 111, 5442 (1989); (d) D.R. Jones, L.F. Lindoy and A.M. Sargeson, ibid, 105, 7327 (1983).
(a) R. Nakon, P.R. Rechani and R.J. Angelici, J. Am. Chem. Soc., 96, 2117 (1974); (b) R.W. Hay, A.K. Basak and M.P. Pujari, J. Chem. Soc., Dalton Trans., 197 (1989); (c) J. Chin and V. Jubian, J. Chem. Soc., Chem. Commun., 839 (1989).
(a) P. Woolley, Nature(London), 258, 677 (1975); (b) P. Woolley, J. Chem. Soc., Perkin Trans. 2, 318 (1977); (c) S.H. Gellman, R. Peter and R. Breslow, J. Am. Chem. Soc., 108, 2388(1986); (d) J.T. Groves Jr. and R. R. Chambers, ibid, 106, 630 (1984); (e) B.L. Iverson and R.A. Lerner, Science, 243, 1185 (1989).
J.E. Coleman, Zinc Enzymes, Birkhauser, Boston, MA, 1986, 49; A. Neuberger and K. Brocklehurst (Eds.), Hydrolytic Enzymes, Elsevier Science, Amsterdam, 1987.
R. L. Fanshawe and A. G. Blackman, Inorg. Chem., 34, 421 (1995).
S. Chen, J.F. Richardson and R.T. Buchanan, Inorg. Chem., 33, 2376 (1994).
L.K. Thompson, B.S. Ramaswamy and E.A. Seymour, Can. J. Chem., 55, 878 (1977).
T. Sakurai, H. Kaji and A. Nakahara, Inorg. Chim. Acta., 67, 1 (1982).
P.J.M. Birker, E.F. Godefroi, J. Helder and J. Reedijk, J. Am. Chem. Soc., 104, 7556 (1982); H.C. Freeman in J. P. Laurent (eds), Coordination Chemistry-21, Pergamon Press, Oxford, 1982, 29.
M.J. Pena, A. Arevalillo, L. Rucandio and J.S. Jimenez, Electrochim, Acta, 35, 673 (1990).
S. Zhu, W. Chen, H. Lin and X. Yin, F. Kou, M. Lin, Y. T. Chen, Polyhedron, 16, 3285 (1997).
W. P. Jencks and M. Gilchrist, J. Am. Chem. Soc., 90, 2622 (1968).
E. Kimura, T. Shiota, T. Koike, M. Shiro, M. Kodama, J. Am. Chem., Soc., 112, 5805 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yin, X., Lin, C., Zhou, Z. et al. Studies of artificial hydrolytic metalloenzymes: The catalyzed carboxyester hydrolysis by copper(II), zinc(II) and cobalt(II) complexes of the tripod ligand tris(2-benzimidazylmethyl)amine. Transition Metal Chemistry 24, 537–540 (1999). https://doi.org/10.1023/A:1006967503458
Issue Date:
DOI: https://doi.org/10.1023/A:1006967503458