Abstract
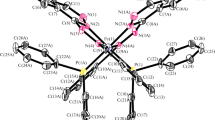
Mononuclear and binuclear chelates of biacetylmonoxime picolinoylhydrazone (H2BMPcH) with CrIII, FeIII, CoII, NiII, CuII, ZnII, CdII, PdII and UO2 2+ have been prepared. Elemental analyses, molar conductivities, spectral (u.v., visible, i.r., n.m.r., e.s.r.), thermal (t.g., d.t.g., d.t.a.) and magnetic susceptibility measurements have been used to characterize the chelates. The i.r. spectral data indicate that H2BMPcH behaves in a bidentate, tridentate and/or tetradentate manner and the hydrazonic azomethine nitrogen constituents the chelating backbone in all chelates. Based on magnetic and spectroscopic data, the structures for the chelates are proposed as follows: tetrahedral for [Co(HBMPcH)(H2O)]Cl, octahedral for [Co(HBMPcH)2], [Cr(HBMPcH)Cl(H2O)]2Cl2, [Fe(HBMPcH)Cl-(H2O)]2Cl2, [Ni(BMPcH)(H2O)2], square-planar for (Ni(HBMPcH)Cl], [Pd(HBMPcH)Cl], [Cu(HBMPcH)(H2O)]Cl and tetragonally distorted octahedral for [Cu(BMPcH)(H2O)2]2 chelates. Generally, the solid metal acetate complexes have a unique decomposition exotherm profile which can be used as a rapid and sensitive tool for the detection of acetate-containing complexes.
Similar content being viewed by others
References
P.W. Ludden, V.K. Shah, G.P. Roberts, M. Homer, R. Allen, T. Paustian, J. Roll, R. Chatterjee, M. Madden and J. Allen, Molybdenum Enzymes, Cofactors and Model Systems, American Chemical Society, Washington DC, 1993, p. 196.
E.M. Page and S.A. Wass, Coord. Chem. Rev., 164, 203 (1997).
B.J. Hales, Adv. Inorg. Biochem., 165 (1990).
K.R. Tsai and H.L. Wan, J. Clust. Sci., 6, 485 (1995).
A. Butler and C.J. Carrano, Coord. Chem. Rev., 109, 61 (1991).
B.M. Nikolova and G. St. Nikolov, J. Inorg. Nucl. Chem., 29, 1013 (1967).
R.H. Dunhill and T.D. Smith, J. Chem. Soc. A, 2189 (1968).
Yu.K. Tselinskii, L.V. Shevcheko and I.I. Kusel'man, Ukr. Khim. Zh., 46, 656 (1980).
P.M. Ehde, I. Anderson and L. Pettersson, Acta Chem. Scand., 143, 136 (1989).
S.G. Vul'fson, A.N. Glebov, O. Yu. Tarasov and Yu. I. Sal'nikov, Dokl. Akad. Nauk. SSSR, 314, 386 (1990). H 11. S.P. Arya and P.K. Sharma, J. Indian Chem. Soc., 69, 793 (1992).
T.A. Dyachkova, R.S. Sa®n, A.N. Glebov and G.K. Budnikov, Zh. Neorg. Khim., 38, 482 (1993).
T. Kiss, P. Buglyo, D. Sanna, G. Micera, P. Decock and D. Dewaele, Inorg. Chim. Acta, 239, 145 (1995).
S. Burojevic, I. Shweky, A. Bino, D.A. Summers and R.C. Thompson, Inorg. Chim. Acta, 251, 75 (1996).
Z.H. Zhou, H.L. Wan, S.Z. Hu and K.R. Tsai, Inorg. Chim. Acta, 237, 193 (1995).
V. Murugesan, V. Babu, S. Sankaran, Inorg. Chem., 37, 1336 (1998).
G.I. Fillin and V.N. Markin, Deposited Doc. VINITI 3475±3479, 162 (1975); Chem. Abstr., 88, 57252e (1976).
C. Djordjevic, M. Lee and E. Sinn, Inorg. Chem., 28, 719 (1989).
D.W. Wright, P.A. Humiston, W.H. Orme-Johnson and W.H. Davis, Inorg. Chem., 34, 4194 (1995).
Z.H. Zhou, W.B. Yan, H.L. Wan, K.R. Tsai, J.Z. Wang and S.Z. Hu, J. Chem. Crystallogr., 25, 807 (1995).
D.W. Wright, R.T. Chang, S.K. Mandal, W.H. Armstrong, W.H.Z.H. Zhou, H.L. Wan and K.R. Tsai, Chinese Sci. Bull., 40, 749 (1995).
J.D. Pedrosa de Jesus, M. de D. Farropas, P. O'Brien, R.D. Gillard and P.A. Williams, Transition Met. Chem., 8, 193 (1983).
L.R. Assunbeni, M.L. Niven, J.J. Cruywagen and J.B. B Heyns, J. Crystallogr. Spectrosc. Res., 17, 373 (1987).
N.W. Alcock, M. Dudek, R. Grybos, E. Hodorowicz, A. Kanas and A. Samotus, J. Chem. Soc., Dalton Trans., 707 (1990).
M.A. Porai-Koshits, L.A. Aslanov, G.V. Ivanova and T.V. Polynova, J. Struct. Chem. (Engl. Transl.), 9, 401 (1968).
J.E. Berg, S. BrandaÈnge, L. Lindblom and P.E. Werner, Acta. Chem. Scand. Ser. A., 31, 325 (1977).
Z.H. Zhou, W.B. Yan, H.L. Wan and K.R. Tsai, Chin. J. Struc. Chem., 14, 255 (1995).
J. Kim and D.C. Rees, Biochem., 33, 389 (1994).
E.L. Muetterties and L.J. Guggenberger, J. Am. Chem. Soc., 96, 1748 (1974).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rakha, T.H. Mononuclear and binuclear chelates of biacetylmonoxime picolinoylhydrazone. Transition Metal Chemistry 24, 659–665 (1999). https://doi.org/10.1023/A:1006936101143
Issue Date:
DOI: https://doi.org/10.1023/A:1006936101143