Abstract
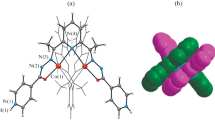
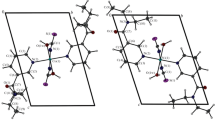
Seven new cobalt(II) complexes based on the Schiff bases, 2,6-diacetylpyridine bis(isonicotinoylhydrazone) (H2L1) and 2,6-diacetylpyridine bis(nicotinoylhydrazone) (H2L2), are synthesized and studied by X-ray diffraction analysis: [Co(H2L1)(NCS)2] · 2.25H2O (I), [Co(H2L2)(NCS)2] · CH3OH (II), [Co(H2L2)(NCS)(H2O)]NCS (III), [Co(H4L1)(NCS)2](NO3)2 · 2H2O (IV), [Co(H4L1)(NCS)2][Co(NCS)4] · 0.75H2O (V), [Co(H4L2)(NCS)2][Co(NCS)4] · 1.75H2O (VI), and [Co(H2L2)(NCS)(CH3OH)]2[Co(NCS)4] · 2CH3OH (VII) (CIF files CCDC 941186 (I), 1457906 (Ia), 1457905 (II), 941187 (III), 1457907 (IV), 1457908 (V), 1457909 (VI), and 941188 (VII)). The organic ligands in the complexes act as pentadentate neutral H2L or doubly protonated (H4L)2+ coordinated through the same set of donor atoms N3O2. In all compounds I–VII, the coordination polyhedron of the Co2+ ion in a complex with the Schiff bases has a shape of a pentagonal bipyramid. The hydrazones are arranged in the equatorial plane of the bipyramid. Its axial vertices are occupied by the nitrogen atoms of the NCS‾ anions in compounds I, II, and IV–VI and by the nitrogen atoms of NCS‾ and oxygen of the water molecule in compound III or methanol in compound VII. The NO -3 anions or [Co(NCS)4]2‾ complex anions obtained by the reactions are involved along with the NCS‾ anions in the formation of compounds IV–VII.
Similar content being viewed by others
References
Davis, M.B., Coord. Chem. Rev., 1997, vol. 164, p. 27.
Smith, D.R., Coord. Chem. Rev., 1998, vol. 172, p. 457.
Radecka-Paryzek, W. and Gdaniec, M., Polyhedron, 1997, vol. 16, no. 20, p. 3681.
Campbell, M.J.M., Coord. Chem. Rev., 1975, vol. 15, nos. 2-3, p. 279.
Williams, D.R., Chem. Rev., 1972, vol. 72, p. 203.
Furst, A. and Haro, R.T., Progr. Exp. Tumor Res., 1969, vol. 12, p. 102.
Sharma, R.P., Kothari, A.K., and Sharma, N.K., Indian J. Derm. Vener. Lepr., 1995, vol. 61, p. 26.
Mazza, P., Orcesi, M., Pelizzi, C., et al., J. Inorg. Biochem., 1992, vol. 48, p. 251.
Allen, F.H., Acta Crystallogr., Sect. B: Struct. Sci., 2002, vol. 58, nos. 3-1, p. 380.
Palenik, G.J. and Wester, D.W., Inorg. Chem., 1978, vol. 17, no. 4, p. 864.
Carcelli, M., Ianelli, S., Pelagatti, P., and Pelizzi, G., Inorg. Chim. Acta, 1999, vol. 292, p. 121.
Qin, S.-N., Chen, Z.-L., Liu, D.-Ch., et al., Transition Met. Chem., 2011, vol. 36, p. 369.
Palenik, G.J., Wester, D.W., Rychlewska, U., and Palenik, R.C., Inorg. Chem., 1976, vol. 15, no. 8, p. 1814.
Gudasi, K.B., Patil, S.A., Vadavi, R.S., et al., Inorg. Chim. Acta, 2006, vol. 359, no. 10, p. 3229.
Neto, B.A.D., Viana, B.F.L., Rodrigues, Th.S., et al., Dalton Trans., 2013, vol. 42, p. 11497.
Constable, E.C., in Comprehensive Supramolecular Chemistry, Sauvage, J.-P., Ed., Oxford: Elsevier, 1996, vol. 9, p. 213.
Konar, S., Jana, A., Das, K., et al., Polyhedron, 2011, vol. 30, no. 17, p. 2801.
Mangia, A., Pelizzi, C., and Pelizzi, G., Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1974, vol. 30, p. 2146.
Ianelli, S., Minardi, G., Pelizzi, C., et al., J. Chem. Soc., Dalton Trans., 1991, p. 2113.
Bonardi, A., Merlo, C., Pelizzi, C., et al., J. Chem. Soc., Dalton Trans., 1991, p. 1063.
Naskar, S., Corbella, M., Blake, A.J., and Chattopadhyay, Sh.K., Dalton Trans., 2007, vol. 11, p. 1150.
Plutenko, M.V., Moroz, Y.S., Sliva, T.Yu., et al., Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2008, vol. 64, p. m137.
Bonardi, A., Ianelli, S., Pelizzi, C., et al., Inorg. Chim. Acta, 1995, vol. 232, nos. 1-2, p. 211.
Batchelor, L.J., Sangalli, M., Guillot, R.G., et al., Inorg. Chem., 2011, vol. 50, p. 12045.
Sessoli, R., Tsai, H.L., Schake, A.R., et al., J. Am. Chem. Soc., 1993, vol. 115, p. 1804.
Bottari, B., Maccari, R., Monforte, F., et al., Bioorg. Med. Chem., 2001, vol. 9, p. 2203.
Bourosh, P., Bulkhak, I., Myrzak, A.I., et al., Koord. Khim., 2016, vol. 42, no. 3, p. 137.
Bulhac, I., Deseatnic-Ciloci, A., Bourosh, P., et al., Chem. J. Mold., 2016, vol. 11, no. 1, p. 39.
CrysAlis RED, O. D. L. Version 1.171.34.76, 2003.
Sheldrick, G., Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, vol. 64, no. 1, p. 112.
Casas, J.S., Garcia-Tasende, M.S., and Sordo, J., Coord. Chem. Rev., 2000, vol. 209, p. 197.
Deroche, A., Morgenstern-Badarau, I., Cesario, M., et al., J. Am. Chem. Soc., 1996, vol. 118, p. 4567.
Lorenzini, C., Pelizzi, C., Pelizzi, G., and Predieri, G., J. Chem. Soc., Dalton Trans., 1983, p. 2155.
Lorenzini, C., Pelizzi, C., Pelizzi, G., and Predieri, G., J. Chem. Soc., Dalton Trans., 1983, p. 721.
Bis, J.A. and Zaworotko, M.J., Cryst. Growth Des., 2005, vol. 5, no. 3, p. 1169.
Cowan, J.A., Howard, J.A.K., McIntyre, G.J., et al., Acta Crystallogr., Sect. B: Struct. Sci., 2003, vol. 59, p. 794.
Bu, X.H., Du, M., Zhang, L., et al., Inorg. Chim. Acta, 2000, vol. 308, p. 143.
Nakamoto, K., Infrared and Raman Spectra of Inorganic and Coordination Compounds, Wiley, 1997.
Nakanisi, K., Infrared Absorption Spectroscopy, San- Francisco Holden Day, 1962.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © I. Bulhac, O. Danilescu, A. Rija, S. Shova, V.Ch. Kravtsov, P.N. Bourosh, 2017, published in Koordinatsionnaya Khimiya, 2017, Vol. 43, No. 1, pp. 23–38.
Rights and permissions
About this article
Cite this article
Bulhac, I., Danilescu, O., Rija, A. et al. Cobalt(II) complexes with pentadentate Schiff bases 2,6-diacetylpyridine hydrazones: Syntheses and structures. Russ J Coord Chem 43, 21–36 (2017). https://doi.org/10.1134/S1070328417010018
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328417010018