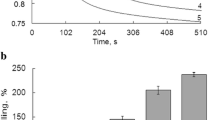
Abstract
The effect of various inhibitors of DNA topoisomerase II, which has been shown to induce apoptotic cell death, on Ca2+ transport in isolated rat liver nuclei was investigated. Ca2+ uptake and release were determined with a Ca2+ electrode. The presence of aurintricarboxylic acid (ATA; 10-6 to 10-4 M), etoposide (10-4 M), genistein (10-5 and 10-4 M) or amsacrine (10-4 M) in the reaction mixture caused a significant increase in Ca2+ release from the nuclei. Also, these compounds (10-4 M) significantly inhibited Ca2+ uptake by the nuclei. However, the presence of ATA (10-5 and 10-4 M) in the enzyme reaction mixture did not significantly inhibit Ca2+-ATPase activity, which is involved in the nuclear Ca2+ uptake, in the liver nuclei, while etoposide (10-4 M), genistein (10-4 M) and amsacrine (10-4 M) appreciably decreased the enzyme activity. Meanwhile, addition of Ca2+ clearly activated DNA fragmentation in the liver nuclei. The Ca2+ activated DNA fragmentation was significantly prevented by the presence of etoposide, genistein and amsacrine with the concentrations of 10-5 and 10-4 M in the reaction mixture, although ATA (10-5 and 10-4 M) had no effect. The present study demonstrates that some apoptosis inducible compounds used can influence on Ca2+ transport system in isolated rat liver nuclei, suggesting a decrease of nuclear Ca2+ level involved in nuclear functions. (Mol Cell Biochem 166: 183-189, 1997)
Similar content being viewed by others
References
Williamson JR, Cooper RH, Hoek JB: Role of calcium in hormonal regulation of liver metabolism. Biochim Biophys Acta 639: 243–295, 1981
Reinhart PH, Taylor WM, Bygrave FL: The role of calcium ions in the mechanisms of action of α-adrenergic agonists in rat liver. Biochem J 223: 1–13, 1984
Boyton AL, Whitfield JF, MacManus JP: Calmodulin stimulates DNA synthesis by rat liver cells. Biochim Biophys Res Commun 95: 745–749, 1980
Cruise J, Houck KA, Michalopoulos GK: Induction of DNA synthesis in cultured rat hepatocytes through stimulation of α-adrenoreceptor by norepinephrine. Nature 227: 749–751, 1985
Backs O, Carafolli E: Calmodulin and calmodulin-binding proteins in liver cell nuclei. J Biol Chem 262: 10786–10790, 1987
Pujol MJ, Soriano M, Alique R, Carafolli E, Backs O: Effect of α-adrenergic blockers on calmodulin association with the nuclear matrix of rat liver cells during proliferative activation. J Biol Chem 264: 18863–18865, 1989
Jones DP, McConkey DJ, Nicotera P, Orrenius S: Calcium-activated DNA fragmentation in rat liver nuclei. J Biol Chem 264: 6398–6403, 1989
Backs O, Lanini L, Serratosa J, Coll MJ, Bastors R, Alique R, Rius E, Carafolli E: Calmodulin-binding proteins in the nuclei of quiescent and proliferatively activated rat liver cells. J Biol Chem 265: 18595–18600, 1990
Nicotera P, McConkey DJ, Jones DP, Orrenius S: ATP stimulates Ca2+ uptake and increase the free Ca2+ concentration in isolated rat liver nuclei. Proc Natl Acad Sci USA 86: 453–457, 1989
Yamaguchi M: Effect of calcium-binding protein regucalcin on Ca2+ transport system in rat liver nuclei: stimulation of Ca2+ release. Mol Cell Biochem 113: 63–70, 1992
Yamaguchi M, Oishi K: Characterization of Ca2+-stimulated adenosine 5′-triphosphatase and Ca2+ sequestering in rat liver nuclei. Mol Cell Biochem 125: 43–49, 1993
Yamaguchi M: A novel Ca2+-binding protein regucalcin and calcium inhibition: Regulatory role in liver cell function. In: K Kohama (ed). Calcium Inhibition. Japan Sci Soc Press, Tokyo and CRC Press, Boca Raton, 1992, pp 19–41
Yamaguchi M, Sakurai T: Inhibitory effect of calcium-binding protein regucalcin on Ca2+-activated DNA fragmentation in rat liver nuclei. FEBS Lett 279: 281–284, 1991
Yamaguchi M, Oishi K: Effect of nuclear Ca2+ uptake inhibitors on Ca2+-activated DNA fragmentation in rat liver nuclei. Mol Cell Biochem 148: 33–37, 1995
Benchokroun Y, Coupric J, Larsen AK: Aurintricarboxylic acid, a putative inhibitor of apoptosis, is a potent inhibitor of DNA topoisomerase II in vitro and in chinese hamster fibrosarcoma cells. Biochem Pharmacol 49: 305–313, 1995
Bina-Stein M, Tritton TR: Aurintricarboxylic acid is a nonspecific enzyme inhibitor. Mol Pharmacol 12: 191–193, 1976
Mizumoto K, Rothman RJ, Farber JL: Programmed cell death (apoptosis) of mouse fibroblasts is induced by the topoisomerase II inhibitor etoposide. Mol Pharmacol 46: 890–895, 1994
Walker PR, Smith C, Youdale T, Leblanc J, Whitfield JF, Sikorska M: Topoisomerase II-reactive chemotherapeutic drugs induce apoptosis in thymocytes. Cancer Res 51: 1078–1085, 1991
Liu Y, Bhalla K, Hill C, Priest DG: Evidence for involvement of tyrosine phosphorylation in taxol-induced apoptosis in a human ovarian tumor cell line. Biochem Pharmacol 48: 1265–1272, 1994
Burton K: A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J 62: 315–323, 1956
Nakamura M, Mori K, Colorimetric determination of inorganic phosphorus in the presence of glucose-1-phosphate and adenosine triphosphate. Nature 182, 1441–1442, 1958
Lowry OH, Rosebrough NJ, Farr AL, Randall FJ: Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–273, 1951
Oishi K, Yamaguchi M: Effect of nicotinamide-adenine dinucleotides on Ca2+ transport system in rat liver nuclei: stimulation of Ca2+ release by NAD+. Mol Cell Biochem 121: 127–133, 1993
Yamaguchi M, Oishi K: Involvement of Ca2+-stimulated adenosine 5′-triphosphatase in the Ca2+ releasing mechanism of rat liver nuclei. Mol Cell Biochem 131: 167–172, 1994
Spinozzi F, Pagliacci MC, Migliorati G, Moraca R, Grignani F, Riccardi C, Nicoletti I: The natural tyrosine kinase inhibitor genistein produces cell cycle arrest and apoptosis in Jurkat T-leukemia cells. Leuk Res 18: 431–439, 1994
Bergamaschi G, Rosti V, Danova M, Ponchio L, Lucotti C, Cazzola M: Inhibitors of tyrosine phosphorylation induce apoptosis in human leukemic cell lines. Leukemia 7: 2012–2018, 1993
McCabe MJ, Orrenius S: Genistein induces apoptosis in immature human thymocytes by inhibiting topoisomerase II. Biochem Biophys Res Commun 194: 944–950, 1993
McConky DJ, Nicotera P, Hartzell P, Ballome G, Wyllie AH, Orreinius S: Glucocorticoids activate an endogeneous suicide process in thymocytes through the elevation of cytosolic Ca2+ concentration. Arch Biochem Biophys 269: 365–370, 1989
McConky DJ, Hartzell D, Duddy SK, Håkansson H, Orreinius S: 2,3,7,8-Tetrachlorodibenzo-p-dioxin kills immature thymocytes by Ca2+-mediated endonuclease activation. Sciences 242: 256–259, 1989
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ueoka, S., Yamaguchi, M. Effect of apoptosis-related compounds on Ca2+ transport system in isolated rat liver nuclei. Mol Cell Biochem 166, 183–189 (1997). https://doi.org/10.1023/A:1006831907937
Issue Date:
DOI: https://doi.org/10.1023/A:1006831907937