Abstract
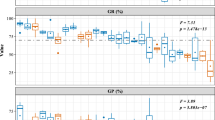
It has been suggested that abortion of ovules in perennials is caused partly by early acting genetic load (abortions due to ‘bad offspring’). However, it is still unclear what proportion of abortions of naturally pollinated seeds are due to early genetic load. Here we suggest that variation between maternal genotypes (abortions due to ‘bad maternal genotypes’) may be an even more important factor causing genetic abortions than early load, based on results from Scots pine. The early load is severe in Scots pine: in experimental self-pollinations on average 76% of the seeds were aborted. Comparison of naturally pollinated and experimentally cross-pollinated seeds showed that the abortion rate of naturally pollinated seeds was only slightly, and not statistically significantly, higher than that of experimentally cross-pollinated seeds (30% vs. 26.5%, respectively). Thus, although early load can be high under self-pollination in Scots pine, it does not account for a high share of abortions of naturally pollinated seeds. Instead, maternal genotype determined the seed abortion rate: in a separate experiment using an experimental population (clonal stand), 29% of the total variance in seed abortion was due to variation between maternal genotypes. We studied further whether ‘bad maternal genotypes’ could be explained by trade-offs between seed abortion and other fitness functions. Only one statistically significant genetic correlation was found, a positive association between cone production and successful seed development. Thus ‘bad maternal genotypes’ aborted a higher proportion of their seed and produced less cones than the ‘good maternal genotypes’.
Similar content being viewed by others
References
Ågren, J. (1988) Between-year variation in flowering and fruit set in frost-prone and frost-sheltered populations of dioecious Rubus chamaemoms. Oecologia 76, 175–183.
Andersson, S. (1993) The potential for selective seed maturation in Achillea ptarmica (Asteraceae). Oikos 66, 36–42.
Bawa, K.S. and Webb, C.J. (1984) Flower, fruit and seed abortion in tropical forest trees: implications for the evolution of paternal and maternal reproductive patterns. Am. J. Bot. 71, 736–751.
Bishir, J. and Namkoong, G. (1987) Unsound seeds in conifers: estimation of numbers of lethal alleles and of magnitudes of effects associated with the maternal parent. Silvae Genetica 36, 180–185.
Burbidge, A.H. and James, S.H. (1991) Postzygotic seed abortion in the genetic system of Stylidium (Angiospermae: Stylidaceae). J. Heredity 82, 319–328.
Casper, B.B. (1984) On the evolution of embryo abortion in the herbaceous perennial Cryptantha (Boraginaceae). Evolution 38, 1337–1349.
Charlesworth, D. (1989) Evolution of low female fertility in plants: pollen limitation, resource allocation and genetic load. Trends Ecol. Evol. 4, 289–292.
Charlesworth, D. and Charlesworth, B. (1987a) The effect of investment in attractive structures on allocation to male and female functions in plants. Evolution 41, 948–968.
Charlesworth, D. and Charlesworth, B. (1987b) Inbreeding depression and its evolutionary consequences. Ann. Rev. Ecol. Syst. 18, 237–268.
Ehrlén, J. (1991) Why do plants produce surplus flowers? A reserve-ovary model. Am. Nat. 138, 918–933.
Falconer, D.S. (1989) Introduction to Quantitative Genetics. Longman, Essex, UK.
Fu, Y.-B. and Ritland, K. (1994) Evidence for the partial dominance of viability genes contributing to inbreeding depression in Mimulus guttatus. Genetics 136, 323–331.
Griffin, A.R. and Lindgren, D. (1985) Effect of inbreeding on production of filled seed in Pinus radiata — experimental results and a model of gene action. Theor. Appl. Genet. 71, 334–343.
Hedrick, P.W. (1987) Genetic load and the mating system in homosporous ferns. Evolution 41, 1282–1289.
Holtsford, T.P. (1985) Nonfruiting hermaphroditic flowers of Calochortus leichtlinii (Liliaceae): potential reproductive functions. Am. J. Bot. 72, 1687–1694.
Houle, D. (1992) Comparing evolvability and variability of quantitative traits. Genetics 130, 195–204.
Johnsson, H. (1976) Contributions to the genetics of empty grains in the seed of Scots pine (Pinus sylvestris). Silvae Genet. 25, 10–15.
Kärkkäinen, K. and Savolainen, O. (1993) The degree of early inbreeding depression determines the selfing rate at the seed stage: model and results from Pinus sylvestris (Scots pine). Heredity 71, 160–166.
Kärkkäinen, K., Koski, V. and Savolainen, O. (1996) Geographical variation in the early inbreeding depression of Scots pine. Evolution 50, 111–119.
Kärkkäinen, K., Kuittinen, H., van Treuren, R., Vogl, C, Oikarinen, S. and Savolainen, O. (1999) Genetic basis of inbreeding depression in Arabis petraea. Evolution 53, 1354–1365.
Kempthorne, O. (1957) An Introduction to Genetic Statistics. The Iowa State University Press, Ames, IA.
Klekowski, E.J. Jr. (1988a) Genetic load and its causes in long-lived plants. Trees 1988, 195–203.
Klekowski, E.J. Jr. (1988b) Progressive cross-and self-sterility associated with aging in fern clones and perhaps other plants. Heredity 61, 247–253.
Klekowski, E.J. Jr. and Godfrey, P.J. (1989) Ageing and mutation in plants. Nature 340, 389–391.
Koski, V. (1970) A study of pollen dispersal as a mechanism of gene flow in conifers. Commun. Inst. For. Fenn. 70.4, 1–78.
Koski, V. (1971) Embryonic lethals of Picea abies and Pinus sylvestris. Commun. Inst. For. Fenn. 75.3, 1–30.
Koski, V. and Muona, O. (1986) Probability of inbreeding in relation to clonal differences in male flowering and embryonic lethals. Proceedings of IUFRO conference on breeding theory, progeny testing and seed orchards, Williamsburgh, Virginia, 13–17 October, 1986, pp. 391–300.
Kozlowski, J. and Stearns, S.C. (1989) Hypotheses for the production of bet-hedging and selective abortion. Evolution 43, 1369–1377.
Krebs, S.L. and Hancock, J.F. (1991) Embryonic genetic load in the highbush blueberry, Vaccinium corymbosum (Ericaceae). Am. J. Bot. 78, 1427–1437.
Morgan, M. (1993) Fruit to flower ratios and trade-offs in size and number. Evol. Ecol. 7, 219–232.
Muona, O. and Harju, A. (1989) Effective population sizes, genetic variability, and mating system in natural stands and seed orchards of Pinus sylvestris. Silvae Genet. 38, 221–228.
Obeso, J.R. (1993) Selective fruit and seed maturation in Asphodelus albus Miller (Liliaceae). Oecologia 93, 564–570.
Pellmyr, O. and Huth, C.J. (1994) Evolutionary stability of mutualism between yuccas and yucca moths. Nature 372, 257–260.
Plym Forshell, C. (1974) Seed development after self-pollination and cross-pollination of Scots pine, Pinus sylvestris L. Studia Forestalia Suecica 118, 1–37.
Roach, D.A. and Wulff, R.D. (1987) Maternal effects in plants. Ann. Rev. Ecol. Syst. 18, 209–235.
Sarvas, R. (1962) Investigation on the flowering and seed crop of Pinus sylvestris. Commun. Inst. For. Fenn. 53.4, 1–198.
SAS Institute (1985) Users's Guide: Statistics. 1985 Edition. SAS Institute, Inc., Gary, NC.
Savolainen, O. (1994) Genetic variation and fitness: conservation lessons from pines. In V. Loeschcke, J. Tomiuk and S.K. Jain (eds) Conservation Genetics, pp. 27–36. Birkhäuser, Basel.
Savolainen, O. Kärkkäinen, K. and Kuittinen, H. (1992) Estimating numbers of embryonic lethals in conifers. Heredity 69, 308–314.
Savolainen, O., Kärkkäinen, K., Harju, A., Nikkanen, T. and Rusanen, M. (1993) Fertility variation in Pinus sylvestris: a test of sexual allocation theory. Am. J. Bot. 80, 1016–1020.
Simmons, M.J. and Crow, J.F. (1977) Mutations affecting fitness in Drosophila populations. Ann. Rev. Genet. 11, 49–78.
Smith, C.C., Hamrick, J.L. and Kramer, C.L. (1990) The advantage of mast years for wind pollination. Am. Nat. 136, 154–166.
Sorensen, F.C. (1969) Embryonic genetic load in coastal Douglas fir, Pseudotsuga menziesii var menziesii. Am. Nat. 103, 389–398.
Stephenson, A.G. (1981) Flower and fruit abortion: proximate causes and ultimate functions. Ann. Rev. Ecol. Syst. 12, 253–279.
Stern, K. (1972) Über die Ergebnisse einiger Versuche zur räumlichen und zeitlichen Verteilung des Pollens einzelner Kiefern. Z. Pflanzenzuchtung 67, 313–326.
Sutherland, S. (1986) Patterns of fruit-set: what controls fruit-flower ratios in plants? Evolution 40, 117–128.
Wiens, D. (1984) Ovule survivorship, brood size, life history, breeding systems, and reproductive success in plants. Oecologia 64, 47–53.
Wiens, D., Calvin, C.L., Wilson, C.A. Davern, C.I., Frank, D. and Seavey, S.R. (1987) Reproductive success, spontaneous embryo abortion, and genetic load in flowering plants. Oecologia 71, 501–509.
Willis, J.H. (1993) Effects of different levels of inbreeding on fitness components in Mimulus guttatus. Evolution 47, 864–876.
Willson, M.F. and Rathcke, B.J. (1974) Adaptive design of the floral display in Asclepias syriaca L. Am. Midl Nat. 92, 47–57.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kärkkäinen, K., Savolainen, O. & Koski, V. Why do plants abort so many developing seeds: bad offspring or bad maternal genotypes?. Evolutionary Ecology 13, 305–317 (1999). https://doi.org/10.1023/A:1006746900736
Issue Date:
DOI: https://doi.org/10.1023/A:1006746900736