Abstract
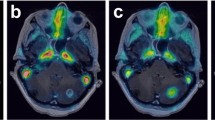
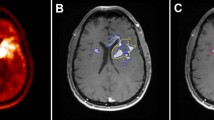
Changes in [18F]-2-fluoro-2-deoxyglucose (FDG) uptake and gadopentetate dimeglumine (Gd-DTPA) enhancement before and after the first course of treatment with a cytostatic agent SU101 (N-[(4-trifluoromethyl)-phenyl]-5-methylisoxazole-4-carboxamide, SUGEN) were assessed using positron emission tomography (PET) and magnetic resonance imaging (MRI) in a pilot study of 8 patients with recurrent supratentorial malignant gliomas. The localization and the volume of Gd-DTPA enhancement and FDG hypermetabolism were analyzed. PET and MRI studies were performed one week before and 7.6±3.7 weeks after administration of SU101. The ratios of mean tumor nobreak activity to mean contralateral white matter and ipsilateral cerebellar activity were calculated for tumor regions, and SUV values corrected to the subjects' body surface area and glucose level (SUVbsa*glu) were calculated for non-tumor regions. Five patients had a substantial increase of tumor volume on both PET and MRI during the first course of SU101. PET and MRI showed roughly equivalent volume changes. Large tumor volume increases were associated with a short time to clinical progression. The metabolic change in the tumor following the first course of SU101 varied from patient to patient, ranging from a 31% reduction to a 43% increase in FDG uptake ratio. Changes in FDG uptake were not predictive of time to progression or survival. In 2 patients with marked clinical deterioration and rapid tumor growth, there were differences in localization of Gd-DTPA enhancement and FDG hypermetabolism suggesting that hypermetabolism beyond the area of contrast enhancement may be of value in predicting rapid progression of high-grade glioma. SU101 did not induce any appreciable changes in SUVbsa*glu for non-tumor brain in 6 of 8 patients.
Similar content being viewed by others
References
Shawver LK, Schwartz DP, Mann E, et al.: Inhibition of platelet-derived growth factor-mediated signal transduction and tumor growth by N-[(4-trifluoromethyl)-phenyl]-5-methylisoxazole-4-carboxamide. Clin Cancer Res 3: 1167-1177, 1997
Williams LT: Signal transduction by the platelet-derived growth factor. Science 243: 1564-1570, 1989
Habenicht AJR, Salbach P, JanBen-Timmen U, Blattner C, Schettler G: Platelet-derived growth-factor-alpha growth factor with an expanding role in health and disease. Klin Wochenschr 68: 53-59, 1990
Plowman G, Ullrich A, Shawver LK: Receptor tyrosine kinases as targets for drug intervention. Drug News and Perspective 7: 334-339, 1994
Cherwinski HM, Byars N, Ballaron SJ, Nakano GM, Young JM, Ranson JT: Leflunomide interferes with pyrimidine nucleotide biosynthesis. Inflamm Res 44: 317-322, 1995
Davis JP, Cain GA, Pitts WJ, Magdola RL, Copeland RA: The immunosuppressive metabolite of leflunomide is a potent inhibitor of human dihydroorotate dehydrogenase. Biochemistry 35: 1270-1273, 1996
Malkin MG, Mason WP, Lieberman FS, et al.: Phase I study of SU101, a novel signal transduction inhibitor, in recurrent malignant glioma. Proc Amer Soc Clin Oncol 16: 385a, 1997
Malkin MG, Rosen L, Lopez AM, et al.: Phase 2 study of SU101, a PDGF-R signal transduction inhibitor, in recurrent malignant glioma. Proc Amer Soc Clin Oncol 17: 390a, 1998
Earnest F, Kelly PJ, Scheithauer BW, et al.: Cerebral astrocytomas: histopathologic correlation of MR and CT contrast enhancement with stereotactic biopsy. Radiology 166: 823-827, 1988
Di Chiro G: Positron emission tomography using [18F]fluorodeoxyglucose in brain tumors. A powerful diagnostic and prognostic tool. Invest Radiol 22: 360-371, 1987
Elster AD, Di Presio DA: Cranial postoperative site: assessment with contrast-enhanced MR imaging. Radiology 174: 93-98, 1990
Di Chiro G, Oldfield E, Wright DC, et al.: Cerebral necrosis after radiotherapy and/or intra-arterial chemotherapy for brain tumors: PET and neuropathologic studies. AJNR 8: 1083-1091, 1987
Alavi JB, Alavi A, Chawluk J, Kushner M, Powe J, Hickey W, Reivich M: Positron emission tomography in patients with glioma. A predictor of prognosis. Cancer 62: 1074-1078, 1988
Delbeke D, Meyerowitz C, Lapidus RL, Maciunas RJ, Jennings MT, Moots PL, Kessler RM: Optimal cutoff levels of F-18 fluorodeoxyglucose uptake in the differentiation of low-grade from high-grade brain tumors with PET. Radiology 195: 47-52, 1995
Davis WK, Boyko OB, Hoffman JM, Hanson MW, Schold SC, Burger PC, Friedman AH, Coleman RE: [18F]2-fluoro-2-deoxyglucose-positron emission tomography correlation of gadolinium-enhanced MR imaging of central nervous system neoplasia. AJNR 14: 515-523, 1993
Gross MW, Weber WA, Feldmann HJ, Bartenstein P, Schwaiger M, Molls M: The value of F-18-fluorodeoxyglucose PET for the 3-D radiation treatment planning of malignant gliomas. Int J Radiat Oncol Biol Phys 41: 989-995, 1998
Olivero WC, Dulebohn SC, Lister JR: The use of PET in evaluating patients with primary brain tumors: is it useful? J Neurol Neurosurg Psychiatry 57: 250-252, 1995
Ricci PE, Karis JP, Heiserman JE, Fram EK, Bice AN, Drayer BP: Differentiating recurrent tumor from radiation necrosis: time for re-evaluation of positron emission tomography? AJNR 19: 407-413, 1998
Hamacher K, Coenen HH, Stocklin G: Efficient stereospecific synthesis of no-carrier-added 2-[18F]-fluoro-2-deoxy-D-glucose using aminopolyether supported nucleophilic substitution. J Nucl Med 27(2): 235-238, 1986
Collignon A, Maes F, Delaere D, Vandermeulen D, Suetens P, Marchal G: Automated multi-modality image registration based on information theory. In: Bizais Y, Barillot C, Di Paola R (eds) Information Processing in Medical Imaging 1995. Kluwer Academic Publishers, Dordrecht, 1995, pp 263-274
Lindholm P, Minn H, Leskinen-Kallio S, Bergman J, Ruotsalainen U, Joensuu H: Influence of the blood glucose concentration on FDG uptake in cancer: a PET study. J Nucl Med 34: 1-6, 1993
Kim CK, Gupta NC, Chandramouli B, Alavi A: Standardized uptake values of FDG: body surface area correction is preferable to body weight correction. J Nucl Med 35: 164-167, 1994
Kole AC, Nieweg OE, Pruim J, Paans AMJ, Plukker JTM, Hoekstra HJ, Koops HS, Vaalburg W: Standardized uptake value and quantification of metabolism for breast cancer imaging with FDG and L-[1-11C]Tyrosine PET. J Nucl Med 38: 692-696, 1997
Di Chiro G, DeLaPaz RL, Brooks RA: Glucose utilization of cerebral gliomas measured by [18]fluorodeoxyglucose and positron emission tomography. Neurology 32: 1323-1329, 1982
Patronas NJ, Di Chiro G, Kufta C, Bairamian D, Kornblith PL, Simon R, et al.: Prediction of survival in glioma patients by means of positron emission tomography. J Neurosurg 62: 816-822, 1985
Mineura K, Yasuda T, Kowada M, Ogawa T, Shishido F, Uemura K: Positron emission tomographic evaluation of radiochemotherapeutic effect on regional cerebral hemocirculation and metabolism in patients with gliomas. J Neuro-Oncol 5: 277-285, 1987
Phillips PC, Dhawan V, Strother SC, Sidtis JJ, Evans AC, Allen JC, Rottenberg DA: Reduced cerebral glucose metabolism and increased brain capillary permeability following high-dose methotrexate chemotherapy: a positron emission tomographic study. Ann Neurol 21: 59-63, 1987
Ogawa T, Uemura K, Shishido F, Yamaguchi T, Murakami M, Inugami A, Kanno I, Sasaki H, Kato T, Hirata K, Kowada M, Mineura K, Yasuda T: Changes of cerebral blood flow, and oxygen and glucose metabolism following radiochemotherapy of gliomas: a PET study. J Comput Assist Tomogr 12: 290-297, 1988
Barker FG, Chang SM, Valk PE, Pounds TR, Prados MD: 18-Fluorodeoxyglucose uptake and survival of patients with suspected recurrent malignant glioma. Cancer 79: 115-126, 1997
Janus TJ, Kim EE, Tilbury R, Bruner JM, Yung WK: Use of [18F]fluorodeoxyglucose positron emission tomography in patients with primary malignant brain tumors. Ann Neurol 33: 540-548, 1993
Schifter T, Hoffman JM, Hanson MW, Boyko OB, Beam C, Paine S, et al.: Serial FDG-PET studies in the prediction of survival in patients with primary brain tumors. J Comput Assist Tomogr 17: 509-516, 1993
Kahn D, Follett KA, Bushnell DL, Nathan MA, Piper JG, Madsen M, et al.: Diagnosis of recurrent brain tumor: value of 201T1 SPECT vs 18F-fluorodeoxyglucose PET. Am J Roentgenol 163: 1459-1465, 1994
Langen KJ, Roosen N, Kuwert T, Herzog H, Kiwit JC, Rota-Kops E, et al.: Early effects of intra-arterial chemotherapy in patients with brain tumors studied with PET: preliminary results. Nucl Med Commun 10: 779-790, 1989
Holzer T, Herholz K, Jeske J, Heiss WD: FDG-PET as a prognostic indicator in radiochemotherapy of glioblastoma. J Comput Assist Tomogr 17: 681-687, 1993
Haberkorn U, Strauss LG, Dimitrakopoulou A, Seiffert E, Oberdorfer F, Ziegler S, Reisser C, Doll J, Helus F, van Kaick G: Fluorodeoxyglucose imaging of advanced head and neck cancer after chemotherapy. J Nucl Med 34: 12-17, 1993
DeWitte O, Hildebrand J, Luxen A, Goldman S: Acute effect of carmustine on glucose metabolism in brain and glioblastoma. Cancer 74: 2836-2842, 1994
Rozental JM, Levine RL, Nickles RJ, Dobkin JA: Glucose uptake by gliomas after treatment: a positron emission tomographic study. Arch Neurol 46: 1302-1307, 1989
Rozental JM, Cohen JD, Mehta MP, Levine RL, Hanson JM, Nickles RJ: Acute changes in glucose uptake after treatment: the effects of carmustine (BCNU) on human glioblastoma multiforme. J Neuro-Oncol 15: 57-66, 1993
Wang L, Hayashi H, Ebina Y: Transient effect of platelet-derived growth factor on GLUT4 translocation in 3T3-L1 adipocytes. J Biol Chem 274: 19 246-19 253, 1999
Conricode KM: Involvement of phosphatidylinositol 3-kinase in stimulation of glucose transport by growth factors in 3T3-L1 adipocytes. Biochem Mol Biol Int 36: 835-843, 1995
Kamohara S, Hayashi H, Todaka M, et al.: Platelet-derived growth factor triggers translocation of the insulinregulatable glucose transporter (type 4) predominantly through phosphatidylinositol 3-kinase binding sites on the receptor. Proc Natl Acad Sci USA 92: 1077-1081, 1995
Plate KH, Breier G, Farrell CL, Risau W: Platelet-derived growth factor receptor-beta is induced during tumor development and upregulated during tumor progression in endothelial cells in human gliomas. Lab Invest 67: 529-534, 1992
Strawn LM, Mann E, Elliger SS, et al.: Inhibition of glioma by a truncated platelet-derived growth factor-beta receptor. J Biol Chem 269: 21 215-21 222, 1994
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Vlassenko, A.G., Thiessen, B., Beattie, B.J. et al. Evaluation of Early Response to SU101 Target-based Therapy in Patients with Recurrent Supratentorial Malignant Gliomas Using FDG PET and Gd-DTPA MRI. J Neurooncol 46, 249–259 (2000). https://doi.org/10.1023/A:1006481313747
Issue Date:
DOI: https://doi.org/10.1023/A:1006481313747