Abstract
Purpose
There is a continuous search for imaging techniques with high sensitivity and specificity for brain tumors. Positron emission tomography (PET) imaging has shown promise, though many PET agents either have a low tumor specificity or impractical physical half-lives. [124I]CLR1404 is a small molecule alkylphosphocholine analogue that is thought to bind to plasma membrane lipid rafts and has shown high tumor-to-background ratios (TBR) in a previous pilot study in brain tumor patients. This study attempts to define the clinical value of [124I]CLR1404 PET/CT (aka CLR124).
Procedures
Adult patients with new or suspected recurrence of high-grade primary or metastatic brain tumors (N = 27) were injected with [124I]CLR1404 followed by PET/CT at 6, 24, and 48 h. Standard uptake values (SUV) and TBR values were calculated for all time points. Uptake of [124I]CLR1404 was qualitatively assessed, compared with magnetic resonance imaging (MRI), and correlated with clinical outcome. Final diagnosis (N = 25) was established based on surgically resected tissue or long-term follow-up.
Results
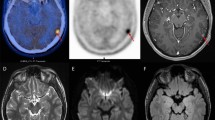
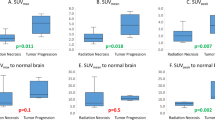
Positive uptake with high TBR was detected in all but one patient with a final diagnosis of primary/recurrent brain tumor (12/13) and in less than half of patients with treatment-related changes (5/12). Concordance between [124I]CLR1404 uptake and contrast enhancement on MRI was seen in < 40 %, with no concordance between T2-hyperintensities and uptake. No significant difference in overall outcome was found between patients with and without [124I]CLR1404 uptake.
Conclusions
The uptake pattern in these patients suggests a very high sensitivity of [124I]CLR1404 PET/CT for diagnosing tumor tissue; however, tumor specificity needs to be further defined. Relative lack of concordance with standard MRI characteristics suggests that [124I]CLR1404 PET/CT provides additional information about brain tumors compared to MRI alone, potentially improving clinical decision-making.






Similar content being viewed by others
References
Kruser TJ, Mehta MP, Robins HI (2013) Pseudoprogression after glioma therapy: a comprehensive review. Expert Rev Neurother 13:389–403
Schwarzenberg J, Czernin J, Cloughesy TF, Ellingson BM, Pope WB, Geist C, Dahlbom M, Silverman DHS, Satyamurthy N, Phelps ME, Chen W (2012) 3′-deoxy-3'-18F-fluorothymidine PET and MRI for early survival predictions in patients with recurrent malignant glioma treated with bevacizumab. J Nucl Med 53:29–36
Goldman S, Pirotte BJ (2011) Brain tumors. Methods Mol Biol 727:291–315
Pirotte BJ, Lubansu A, Massager N et al (2010) Clinical impact of integrating positron emission tomography during surgery in 85 children with brain tumors. J Neurosurg Pediatr 5:486–499
Pirotte BJ, Levivier M, Goldman S et al (2009) Positron emission tomography-guided volumetric resection of supratentorial high-grade gliomas: a survival analysis in 66 consecutive patients. Neurosurgery 64:471–481
Gulyas B, Halldin C (2012) New PET radiopharmaceuticals beyond FDG for brain tumor imaging. Q J Nucl Med Mol Imaging 56:173–190
Albert NL, Weller M, Suchorska B, Galldiks N, Soffietti R, Kim MM, la Fougère C, Pope W, Law I, Arbizu J, Chamberlain MC, Vogelbaum M, Ellingson BM, Tonn JC (2016) Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro-Oncology 18:1199–1208
Calabria F, Cascini GL (2015) Current status of 18F-DOPA PET imaging in the detection of brain tumor recurrence. Hell J Nucl Med 18:152–156
Wray R, Solnes L, Mena E (2015) 18F-Flourodeoxy-glucose PET/computed tomography in brain tumors: value to patient management and survival outcomes. PET Clin 10:423–430
Tomura N, Mizuno Y, Saginoya T (2016) PET/CT findings for tumors in the base of the skull: comparison of 18F-FDG with 11C-methionine. Acta Radiol 57:325–332
Li Z, Yu Y, Zhang H, Xu G, Chen L (2015) A meta-analysis comparing 18F-FLT PET with 18F-FDG PET for assessment of brain tumor recurrence. Nucl Med Commun 36:695–701
Lapa C, Linsenmann T, Monoranu CM, Samnick S, Buck AK, Bluemel C, Czernin J, Kessler AF, Homola GA, Ernestus RI, Lohr M, Herrmann K (2014) Comparison of the amino acid tracers 18F-FET and 18F-DOPA in high-grade glioma patients. J Nucl Med 55:1611–1616
Lizarraga KJ, Allen-Auerbach M, Czernin J et al (2014) 18F-FDOPA PET for differentiating recurrent or progressive brain metastatic tumors from late or delayed radiation injury after radiation treatment. J Nucl Med 55:30–36
Filss CP, Galldiks N, Stoffels G, Sabel M, Wittsack HJ, Turowski B, Antoch G, Zhang K, Fink GR, Coenen HH, Shah NJ, Herzog H, Langen KJ (2014) Comparison of 18F-FET PET and perfusion-weighted MR imaging: a PET/MR imaging hybrid study in patients with brain tumors. J Nucl Med 55:540–545
Pinchuk AN, Rampy MA, Longino MA, Skinner RWS, Gross MD, Weichert JP, Counsell RE (2006) Synthesis and structure-activity relationship effects on the tumor avidity of radioiodinated phospholipid ether analogues. J Med Chem 49:2155–2165
Weichert JP, Clark PA, Kandela IK, Vaccaro AM, Clarke W, Longino MA, Pinchuk AN, Farhoud M, Swanson KI, Floberg JM, Grudzinski J, Titz B, Traynor AM, Chen HE, Hall LT, Pazoles CJ, Pickhardt PJ, Kuo JS (2014) Alkylphosphocholine analogs for broad-spectrum cancer imaging and therapy. Sci Transl Med 6:240ra75
Swanson KI, Clark PA, Zhang RR, Kandela IK, Farhoud M, Weichert JP, Kuo JS (2015) Fluorescent cancer-selective alkylphosphocholine analogs for intraoperative glioma detection. Neurosurgery 76:115–124
Zhang RR, Swanson KI, Hall LT, Weichert JP, Kuo JS (2016) Diapeutic cancer-targeting alkylphosphocholine analogs may advance management of brain malignancies. CNS Oncol 5:223–231
Clark PA, Al-Ahmad AJ, Qian T et al (2016) Analysis of cancer-targeting alkylphosphocholine analogue permeability characteristics using a human induced pluripotent stem cell blood-brain barrier model. Mol Pharm 13:3341–3349
Hall LT, Titz B, Robins HI et al (2017) Am J Nucl Med Mol Imaging 7:157–166
Acknowledgements
We gratefully acknowledge our dedicated clinical study coordinators (Diana Trask, Nick Anger, Lori Hayes, and Belinda Buehl-Soppe), PET study coordinator (Christine Jaskowiak), statistician (Kaitlin Woo), and of course our patient volunteers who selflessly gave their time and trust to participate in our studies.
Funding
This study was funded by grants UL1TR000427, P30CA014520, and R01CA158800.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All authors declare that he/she has no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hall, L.T., Titz, B., Baidya, N. et al. [124I]CLR1404 PET/CT in High-Grade Primary and Metastatic Brain Tumors. Mol Imaging Biol 22, 434–443 (2020). https://doi.org/10.1007/s11307-019-01362-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-019-01362-1