Abstract
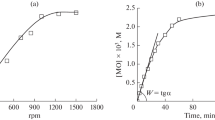
The liquid-phase oxidation of pinane to pinane hydroperoxide (PHP) by dioxygen is studied at 353–373 K and under dioxygen pressures ranging from 2 to 4 atm. The rate of pinane oxidation is described by the equation w = w O 2 + w PHP , where the term w O 2 is independent of the PHP concentration, w PHP is a function of the concentration [PHP]n, and n ranges from 0.5 at low [PHP] to 1.0 at high [PHP]. The cis-isomer of 2-hydroperoxopinane generates free radicals during autooxidation. The ratio of the rate constants of cis- and trans-pinane oxidation is found (k cis /k trans = 4.0). The selectivity of the oxidation of pinane to PHP can reach 90–95%.
Similar content being viewed by others
References
Schmidt-Renner, W., Miffeilungsblatt Chemische Gesellschaft der DDR, 1986, vol. 33, no. 8, p. 175.
Pugni, P., Tecnol. Chim., 1988, vol. 8, no. 11, p. 156.
Fisher, G.S., Goldblat, L.A., Kniel, L., and Snyder, A.D., Ind. Eng. Chem., 1951, vol. 43, p. 671.
Brose, T., Pritzkow, W., and Thomas, G., J. Pract. Chem., 1992, vol. 334, p. 403.
Fisher, G.S., Stinson, J.S., and Goldblat, L.A., J. Am. Chem. Soc., 1953, vol. 75, no. 15, p. 3675.
Fisher, G.S., Stinson, J.S., Moore, R.N., and Goldblat, L.A., Ind. Eng. Chem., 1955, vol. 47, p. 1368.
Sercheli, R., Ferreira, A.L.B., Bartistella, L.H.B., and Schuchardt, U., J. Agric., Food Chem., 1997, vol. 45, no. 4, p. 1361.
US Patent 3723542, 1977.
Il'ina, I.I., Simakova, I.L., and Semikolenov, V.A., Kinet. Katal. (in press).
Rakhimov, A.I., Khimiya i tekhnologiya organicheskikh perekisnykh soedinenii (Chemistry and Technology of Organic Peroxides), Moscow: Khimiya, 1979.
Emanuel', N.M. and Knorre, D.G., Kurs khimicheskoi kinetiki (Chemical Kinetics), Moscow: Vysshaya Shkola, 1962, p. 290.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Il'ina, I.I., Simakova, I.L. & Semikolenov, V.A. Kinetics of Pinane Oxidation to Pinane Hydroperoxide by Dioxygen. Kinetics and Catalysis 42, 41–45 (2001). https://doi.org/10.1023/A:1004896512191
Issue Date:
DOI: https://doi.org/10.1023/A:1004896512191