Abstract
The results of studying the reaction kinetics of the catalytic oxidation of methyl oleate with hydrogen peroxide in a two-phase system (aqueous phase-organic phase) in the presence of the bifunctional catalyst [(Octn)3NMe]3{PO4[WO(O2)2]4} are presented. For the selected reaction conditions, the first orders of reaction with respect to the catalyst, substrate, and oxidizing agent were established. The activation energy was 47 ± 3 kJ/mol in a temperature range of 313–353 K, and the preexponential factor was (6.0 ± 0.3) × 107 L2 mol–2 min–1.



Similar content being viewed by others
REFERENCES
Cerone, M. and Smith, T.K., Front. Nutr., 2021, vol. 8, p. 1.
Biermann, U., Bornscheuer, U.T., Feussner, I., Meier, M.A.R., and Metzger, J.O., Angew. Chem. Int. Ed., 2021, vol. 60, no. 37, p. 20144.
Otopkova, K.V., Esipovich, A.L., Kanakov, E.A., Charykova, T.A., Baidachenko, V.E., and Ryabova, T.A., Kinet. Katal., 2022, vol. 63, no. 6.
Singh, H. and Ali, A., Kinet. Katal., 2022, vol. 63, no. 6.
Belousov, A.S., Esipovich, A.L., Kanakov, E.A., and Otopkova, K.V., Sust. Energy Fuels, 2021, vol. 5, no. 18, p. 4512.
Wypych, G., PVC Degradation and Stabilization, ChemTec Publishing, 2015, p. 287.
Meng, Y., Taddeo, F., Aguilera, A.F., Cai, X., Russo, V., Tolvanen, P., and Leveneur, S., Catalysts, 2021, vol. 11, no. 7, p. 765.
Swern, D., Findley, T.W., and Scanlan, J.T., J. Am. Chem. Soc., 1944, vol. 66, no. 11, p. 1925.
Musik, M., Janus, E., Pelech, R., and Salaciński, L., Catalysts, 2021, vol. 11, no. 9, p. 1058.
Esipovich, A.L., Belousov, A.S., Kanakov, E.A., Mironova, V.Yu., Rogozhin, A.E., Danov, S.M., Vorotyntsev, A.V., and Makarov, D.A., Kinet. Catal., 2019, vol. 60, no. 1, p. 62.
Pai, Z.P., Chesalov, Y.A., Berdnikova, P.V., Uslamin, E.A., Yushchenko, D.Y., Uchenova, Y.V., Khlebnikova, T.B., Baltakhinov, V.P., Kochubey, D.I., and Bukhtiyarov, V.I., Appl. Catal. A: Gen., 2020, vol. 604, p. 117786.
Khlebnikova, T.B., Pai, Z.P., Fedoseeva, L.A., and Mattsat, Y.V., React. Kinet. Catal. Lett., 2009, vol. 98, no. 1, p. 9.
Pai, Z.P., Khlebnikova, T.B., Mattsat, Y.V., and Parmon, V.N., React. Kinet. Catal. Lett., 2009, vol. 98, no. 1, p. 1.
Maiti, S.K., Snavely, W.K., Venkitasubramanian, P., Hagberg, E.C., Busch, D.H., and Subramaniam, B., Ind. Eng. Chem. Res., 2019, vol. 58, no. 7, p. 2514.
Yushchenko, D.Y., Pai, Z.P., and Khlebnikova, T.B., Catal. Lett., 2022, vol. 152, no. 7, p. 2025.
Sheldon, R.A., Arends, I.W.C.E., and Dijksman, A., Catal. Today, 2000, vol. 57, nos. 1–2, p. 157.
Funding
This work was carried out within the framework of a state contract of the Boreskov Institute of Catalysis, Siberian Branch, Russian Academy of Sciences (project no. AAAA-A21-121011390007-7).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by V. Makhlyarchuk
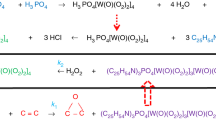
Abbreviations and notation: MO, methyl oleate; Cat, (N-methyl-N,N,N-trioctyl)-tetrakisoxodiperoxotungstophosphate [(Octn)3-NMe]3{PO4[WO(O2)2]4}; concentration, mol/L; T, temperature, K; t, reaction time, min; W, rate of reaction, mol L–1 min–1; rpm, revolutions per minute; A, preexponential factor, L2 mol–2 min–1; l, order of reaction with respect to the catalyst [(Octn)3NMe]3{PO4[WO(O2)2]4}; m, order of reaction with respect to the oxidizing agent H2O2; p, order of reaction with respect to the substrate, MO; Ea, apparent activation energy, kJ/mol; kobs, reaction rate constant of the formation of methyl oleate epoxide, L mol–1 min–1; FA, fatty acid; MOE, methyl oleate epoxide
Rights and permissions
About this article
Cite this article
Yushchenko, D.Y., Pai, Z.P., Uchenova, Y.V. et al. Kinetics of the Catalytic Oxidation of Methyl Oleate. Kinet Catal 64, 270–275 (2023). https://doi.org/10.1134/S0023158423030102
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0023158423030102