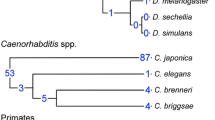
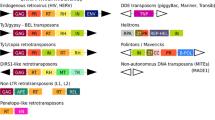
Abstract
Model organisms have proved to be highly informative for many types of genetic studies involving ‘conventional’ genes. The results have often been successfully generalized to other closely related organisms and also, perhaps surprisingly frequently, to more distantly related organisms. Because of the wealth of previous knowledge and their availability and convenience, model organisms were often the species of choice for many of the earlier studies of transposable elements. The question arises whether the results of genetic studies of transposable elements in model organisms can be extrapolated in the same ways as those of conventional genes? A number of observations suggest that special care needs to be taken in generalizing the results from model organisms to other species. A hallmark of many transposable elements is their ability to amplify rapidly in species genomes. Rapid spread of a newly invaded element throughout a species range has also been demonstrated. The types and genomic copy numbers of transposable elements have been shown to differ greatly between some closely related species. Horizontal transfer of transposable elements appears to be more frequent than for nonmobile genes. Furthermore, the population structure of some model organisms has been subject to drastic recent changes that may have some bearing on their transposable element genomic complements. In order to initiate discussion of this question, several case studies of transposable elements in well-studied Drosophila species are presented.
Similar content being viewed by others
References
Anxolabéhère, D., M.G. Kidwell & G. Périquet, 1988. Molecular characteristics of diverse populations are consistent with the hypothesis of a recent invasion of Drosophila melanogaster by mobile P elements. Mol. Biol. Evol. 5: 252–269.
Barakat, A., G. Matassi & G. Bernardi, 1998. Distribution of genes in the genome of Arabidopsis thaliana and its implications for the genome organization of plants. Proc. Natl. Acad. Sci. USA 95: 10044–10049.
Biemont, C. & G. Cizeron, 1999. Distribution of transposable elements in Drosophila species. Genetica 105: 43–62.
Biemont, C., S. Ronsseray, D. Anxolabéhère, H. Izaabel & C. Gautier, 1990. Localization of P elements, copy number regulation, and cytotype determination in Drosophila melanogaster. Genet. Res. 56: 3–14.
Biemont, C., A. Tsitrone, C. Vieira & C. Hoogland, 1997. Transposable element distribution in Drosophila. Genetics 147: 1997–1999.
Blackman, R., R. Grimaila, M. Koehler & W. Gelbart, 1987. Mobilization of hobo elements residing within the decapentaplegic gene complex: suggestion of a new hybrid dysgenesis system in Drosophila melanogaster. Cell. 49: 497–505.
Bucheton, A., R. Paro, H.M. Sang, A. Pelisson & D.J. Finnegan, 1984. The molecular basis of I-R hybrid dysgenesis: identification, cloning and properties of the I factor. Cell. 38: 153–163.
Bucheton, A., C. Vaury, M.C. Chaboissier, P. Abad, A. Pelisson & M. Simonelig, 1992. I elements and the Drosophila genome. Genetica 86: 175–190.
Caceres, M., J.M. Ranz, A. Barbadilla, M. Long & A. Ruiz, 1999. Generation of a widespread Drosophila inversion by a transposable element. Science 285: 415–418.
Charlesworth, B., C.H. Langley & P.D. Sniegowski, 1997. Transposable element distributions in Drosophila. Genetics 147: 1993–1995.
Clark, J.B., T.K. Altheide, M.J. Schlosser & M.G. Kidwell, 1995. Molecular evolution of P transposable elements in the genus Drosophila. I. The saltans and willistoni species groups. Mol. Biol. Evol. 12: 902–913.
Clark, J.B. & M.G. Kidwell, 1997. A phylogenetic perspective on P transposable element evolution in Drosophila. Proc. Natl. Acad. Sci. USA 94: 11428–11433.
Daniels, S.B., K.R. Peterson, L.D. Strausbaugh, M.G. Kidwell & A. Chovnick, 1990. Evidence for horizontal transmission of the P transposable element between Drosophila species. Genetics 124: 339–355.
Dowsett, A.P. & M.W. Young, 1982. Differing levels of dispersed repetitive DNA among closely related species of Drosophila. Proc. Natl. Acad. Sci. USA 79: 4570–4574.
Ehrman, L. & J.R. Powell, 1982. The Drosophila willistoni species group, pp. 193–225, in The Genetics and Biology of Drosophila, Vol. 3b, edited by M. Ashburner, H.L. Carson and J.N. Thompson, Academic Press, London.
Engels, W.R., 1992. The origin of P elements in Drosophila melanogaster. Bioessays 14: 681–686.
Evgen'ev, M., H. Zelentsova, L. Mnjoian, H. Poluectova & M.G. Kidwell, 2000. Invasion of Drosophila virilis by the Penelope transposable element. Chromosoma (In Press).
Evgen'ev, M.B., G.N. Yenikolopov, N.I. Peunova & Y.V. Ilyin, 1982. Transposition of mobile genetic elements in interspecific hybrids of Drosophila. Chromosoma 85: 375–386.
Evgen'ev, M.B., H. Zelentsova, N. Shostak, M. Kozitsina, V. Barsky, D.-H. Lankenau & V.G. Corces, 1997. Penelope, a new family of transposable elements and its possible role in hybrid dysgenesis in Drosophila virilis. Proc. Natl. Acad. Sci. USA 94: 196–201.
Fujii, S., 1942. Further studies on the salivary gland chromosomes of Drosophila virilis. Cytologia 12: 435–459.
Gloor, G.B., C.R. Preston, D.M. Johnson-Schlitz, N.A. Nassif, R.W. Phillis, W.K. Benz, H.M. Robertson & W.R. Engels, 1993. Type I repressors of P element mobility. Genetics 135: 81–95.
Gubenko, I.S. & M.B. Evgen'ev, 1984. Cytological and linkage maps of Drosophila virilis chromosomes. Genetica 65: 127–139.
Hagemann, S., E. Haring & W. Pinsker, 1996. Repeated horizontal transfer of P transposons between Scaptomyza pallida and Drosophila bifasciata. Genetica 98: 43–51.
Hsu, T.C., 1952. Chromosomal variation and evolution in the virilis group of Drosophila. Univ. Texas Pub. 5204: 35–72.
Hyytia, P., P. Capy, J.R. David & R.S. Singh, 1985. Enzymatic and quantitative variation in European and African populations of Drosophila simulans. Heredity (Edinburgh) 54: 209–217.
Jordan, I.K., L.V. Matyunina & J.F. McDonald, 1999. Evidence for the recent horizontal transfer of long terminal repeat retrotransposon. Proc. Natl. Acad. Sci. USA 96: 12621–12625.
Kazazian, H.H., Jr. & J.V. Moran, 1998. The impact of L1 retro-transposons on the human genome. Nat. Genet. 19: 19–24.
Kidwell, M.G., 1983. Evolution of hybrid dysgenesis determinants in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 80: 1655–1659.
Kidwell, M.G., 1993. Lateral transfer in natural populations of eukaryotes. Ann. Rev. Genet. 27: 235–256.
Kidwell, M.G., 1994a. The evolutionary history of the P family of transposable elements. J. Heredity 85: 339–346.
Kidwell, M.G., 1994b. The Wilhelmine E. Key 1991 Invitational Lecture. The evolutionary history of the P family of transposable elements. J. Hered 85: 339–346.
Kidwell, M.G., J.F. Kidwell & J.A. Sved, 1977. Hybrid dysgenesis in Drosophila melanogaster: A syndrome of aberrant traits including mutation, sterility & male recombination. Genetics 36: 813–833.
Kim, J.M., S. Vanguri, J.D. Boeke, A. Gabriel & D.F. Voytas, 1998. Transposable elements and genome organization: A comprehensive survey of retrotransposons revealed by the complete Saccharomyces cerevisiae genome sequence. Genome Res. 8: 464–478.
Kimura, K. & M.G. Kidwell, 1994. Differences in P element population dynamics between the sibling species Drosophila melanogaster and Drosophila simulans. Genet. Res. 63: 27–38.
Kordis, D. & F. Gubensek, 1998. Unusual horizontal transfer of a long interspersed nuclear element between distant vertebrate classes. Proc. Natl. Acad. Sci. USA 95: 10704–10709.
Koshland, D.E., 1988. Biological systems. Science 240: 1385.
Langley, C.H., E. Montgomery, R. Hudson, N. Kaplan & B. Charlesworth, 1988. On the role of unequal exchange on the containment of transposable element copy number. Genet. Res. 52: 223–235.
Lozovskaya, E.R., V.S. Scheinker & M.B. Evgen'ev, 1990. A hybrid dysgenesis syndrome in Drosophila virilis. Genetics 126: 619–623.
O'Hare, K. & G.M. Rubin, 1983. Structures of P transposable elements and their sites of insertion and excision in the Drosophila melanogaster genome. Cell. 34: 25–35.
Patterson, J.T., 1941. The virilis group of Drosophila in Texas. Amer. Nat. 75: 523–529.
Petrov, D.A., J.L. Schutzman, D.L. Hartl & E.R. Lozovskaya, 1995. Diverse transposable elements are mobilized in hybrid dysgenesis in Drosophila virilis. Proc. Natl. Acad. Sci. 92: 8050–8054.
Regner, L.P., M.S. Pereira, C.E. Alonso, E. Abdelhay & V.L. Valente, 1996. Genomic distribution of P elements in Drosophila willistoni and a search for their relationship with chromosomal inversions. J. Hered 87: 191–198.
Robertson, H.M., 1997. Multiple mariner transposons in flatworms and hydras are related to those of insects. J. Hered. 88: 195–201.
Roy, A.M., M.L. Carroll, D.H. Kass, S.V. Nguyen, A.-H. Salem, M.A. Batzer & P.L. Deininger, 1999. Recently integrated human Alu repeats: finding needles in the haystack. Genetica 107: 149–161.
SanMiguel, P., B.S. Gaut, A. Tikhonov, Y. Nakajima & J.L. Bennetzen, 1998. The paleontology of intergene retrotransposons of maize. Nat. Genet. 20: 43–45.
SanMiguel, P., A. Tikhonov, Y.K. Jin, N. Motchoulskaia, D. Zakharov, A. Melake-Berhan, P.S. Springer & K.J. Edwards, 1996. Nested retrotransposons in the intergenic regions of the maize genome. Science 274: 765–768.
Scheinker, S.V., E.R. Lozovskaya, J.G. Bishop, V.G. Corces & M.B. Evgen'ev, 1990. A long terminal repeat-containing retrotransposon is mobilized during hybrid dysgenesis in Drosophila virilis. Proc. Natl. Acad. Sci. USA 87: 9615–9619.
Simmons, G.M., 1992. Horizontal transfer of hobo transposable elements with in the Drosophila melanogaster species complex: evidence from DNA sequencing. Mol. Biol. Evol. 9: 1050–1060.
Singh, R.S., M. Choudhary & J.R. David, 1987. Contrasting patterns of geographic variation in the cosmopolitan sibling species Drosophila melanogaster and Drosophila simulans. Biochem. Genet. 25: 27–40.
Vieira, C., D. Lepetit, S. Dumont & C. Biemont, 1999. Wake up of transposable elements following Drosophila simulans worldwide colonization. Mol. Biol. Evol. 16: 1251–1255.
Vieira, J., C.P. Vieira, D.L. Hartl & E.R. Lozovskaya, 1998. Factors contributing to the hybrid dysgenesis syndrome in Drosophila virilis. Genet. Res. 71: 109–117.
Warters, M., 1944. Chromosomal aberrations in wild populations of Drosophila. Univ. Texas Pub. 4445: 129–174.
Wasserman, M. & F. Wasserman, 1992. Polymorphism in island species of Drosophila. Evol. Biol. 26: 351–381.
Waterston, R. & J. Sulston, 1995. The genome of Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 92: 10836–10840.
Wright, D.A., N. Ke, J. Smalle, B.M. Hauge, H.M. Goodman & D.F. Voytas, 1996. Multiple non-LTR retrotransposons in the genome of Arabidopsis thaliana. Genetics 142: 569–578.
Zelentsova, H., H. Poluectova, L. Mnjoian, G. Lyozin, V. Veleikod-vorskaja, L. Zhivotovsky, M.G. Kidwell & M.B. Evgen'ev, 1999. Distribution and evolution of mobile elements in the virilis species group of Drosophila. Chromosoma 108: 443–456.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kidwell, M.G., Evgen'ev, M.B. How valuable are model organisms for transposable element studies?. Genetica 107, 103–111 (1999). https://doi.org/10.1023/A:1003933419159
Issue Date:
DOI: https://doi.org/10.1023/A:1003933419159