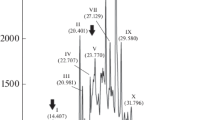
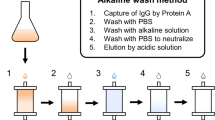
Abstract
A new and rapid procedure has been developed for the isolation of the bacteriochlorophyll a-containing Fenna—Matthews—Olson (FMO)-protein from green sulfur bacteria. Polyclonal antibodies raised against the FMO-protein of Chlorobium (Chl.) tepidum were employed in the preparation of an antibody column utilizing immobilized protein A as the matrix. The antibody column afforded essentially a one-step purification process, resulting in preparations that were free from contaminating pigments and proteins. This was evidenced by absorption spectroscopy, SDS—PAGE, and fluorescence emission.
Similar content being viewed by others
References
Blankenship RE, Cheng P, Causgrove TP, Brune DC, Wang SH, Choh JU and Wang J (1993) Redox regulation of energy transfer efficiency in antennas of green photosynthetic bacteria. Photochem Photobiol 57: 103–107
Blankenship RE, Olson JM and Miller M (1995) Antenna complexes from green photosynthetic bacteria. In: Blankenship RE, Madigan MT and Bauer CE (eds) Anoxygenic Photosynthetic Bacteria, pp 399–435. Kluwer Academic Publishers, Dordrecht, The Netherlands
Blankenship RE, Wang J, Causgrove TP and Brune DC (1990) Efficiency and kinetics of energy transfer in chlorosome antennas from green photosynthetic bacteria. In: Baltscheffsky M (ed) Current Research in Photosynthesis, Vol II, pp 17–24. Kluwer Academic Publishers, Dordrecht, The Netherlands
Buck DR, Savikhin S and Struve WS (1997) Ultrafast absorption difference spectra of the Fenna-Matthews-Olson protein at 19K: Experiment and simulations. Biophys J 72: 24–36
Daurat-Larroque ST, Brew K and Fenna RE (1986) The complete amino acid sequence of a bacteriochlorophyll a-protein from Prosthecochloris aestuarii. J Biol Chem 261: 3607–3615
Dracheva S, Williams JC and Blankenship RE (1992) Cloning and sequencing of the FMO-protein gene from Chlorobium tepidum. In: Murata N (ed) Research in Photosynthesis, Vol I, pp 53–56. Kluwer Academic Publishers, Dordrecht, The Netherlands
Feiler U and Hauska G (1995) The reaction center from green sulfur bacteria. In: Blankenship RE, Madigan MT and Bauer CE (eds) Anoxygenic Photosynthetic Bacteria, Vol II, pp 665–685. Kluwer Academic Publishers, Dordrecht, The Netherlands
Francke C and Amesz J (1997) Isolation and pigment composition of the antenna system of four species of green sulfur bacteria. Photosynth Res 52: 137–146
Franken EM, Neerken S, Louwe RJ, Amesz J and Aartsma TJ (1998) A permanent hole burning study of the FMO antenna complex of the green sulfur bacterium Prothecochloris aestuarii. Biochemistry 37: 5046–51
Gosh AK and Olson JM (1968) Effects of denaturants on the absorption spectrum of the bacteriochlorophyll-protein from the photosynthetic bacterium Chloropseudomonas ethylicum. Biochim Biophys 162: 135–148
Griesbeck C, Hager-Braun C, Rogl H and Hauska G (1998) Quantitation of P840 reaction center preparations from Chlorobium tepidum: Chlorophylls and FMO-protein. Biochim Biophys 1365: 285–293
Johnson SG and Small GJ (1991) Excited-state structure and energy-transfer dynamics of the bacteriochlorophyll a antenna complex from Prosthecochloris aestuarii. J Phys Chem 95: 471–479
Li YF, Zhou WL, Blankenship RE and Allen JP (1997) Crystal structure of the bacteriochlorophyll a protein from Chlorobium tepidum. J Mol Biol 271: 456–471
Lindmark R, Thoren-Tolling K and Sjoquist J (1983) Binding of immunoglobulins to protein A and immunoglobulin levels in mammalian sera. J Immunol Meth 62: 1–13
Lu XY and Pearlstein RM (1993) Simulations of Prosthecochloris bacteriochlorophyll a-protein optical spectra improved by parametric computer search. Photochem Photobiol 57: 86–91
Matthews BW and Fenna R (1980) Structure of a green bacteriochlorophyll protein. Acc Chem Res 13: 309–317
Miller M, Cox RP and Olson JM (1994) Low temperature spectroscopy of isolated FMO-protein and a membrane-free reaction center complex from the green sulfur bacterium Chlorobium tepidum. Photosynth Res 41: 97–100
Olson JM (1998) Chlorosome origin and function in green photosynthetic bacteria. Photochem Photobiol 67: 61–75
Olson JM (1978a) Bacteriochlorophyll a-proteins of two green photosynthetic bacteria. Meth Enzymol 69: 336–344
Olson JM (1978b) Bacteriochlorophyll a-proteins from green bacteria. In: Clayton RK and Sistrom WR (eds) The Photosynthetic Bacteria, pp 161–178. Plenum Press, New York
Pearlstein RM (1992) Theory of the optical spectra of the bacteriochlorophyll a antenna protein trimer from Prosthecochloris aestuarii. Photosynth Res 31: 213–226
Peterson GL (1977) A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem 83: 346–356
Schägger H and von Jagow G (1987) Tricine sodium dodecyl-sulfate polyacrylamide-gel electrophoresis for the separation of proteins in the range from 1 kDa to 100 kDa. Anal Biochem 166: 368–379
Sisson TH and Castor CW (1990) An improved method for immobilizing IgG antibodies on protein A-agarose. J Immunol Meth 127: 215–220
Tronrud DE and Matthews BW (1993) Refinement of the Structure of aWater-Soluble Antenna Complex from Green Photosynthetic Bacteria by Incorporation of the Chemically Determined Amino Acid Sequence. In: Norris J and Deisenhofer J (eds) The Photosynthetic Reaction Center, Vol 1, pp 13–21 Academic Press, San Diego, CA
Tronrud DE, Schmid MF and Matthews BW (1986) Structure and X-ray amino acid sequence of a bacteriochlorophyll a protein from Prosthecochloris refined at 1.9Å resolution. J Mol Biol 188: 443–454
Vulto SIE, Streltsov AM and Aartsma TJ (1997) Excited state energy relaxation in the FMO complexes of the green bacterium Prosthecochloris aestuarii at low temperatures. J Phys Chem B 101: 4845–4850
Wahlund TM, Woese CR, Castenholz RW and Madigan MT (1991) A thermophilic green sulfur bacterium from New Zealand hot springs, Chlorobium tepidum sp. Nov. Arch Microbiol 156: 81–90
Zhou W, LoBrutto R, Lin S and Blankenship RE (1994) Redox effects on the bacteriochlorophyll a-containing Fenna-Matthews-Olson protein from Chlorobium tepidum. Photosynth Res 41: 89–96
Zhou W (1995) Ph.D Thesis, Arizona State University, Tempe, AZ
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, D.D., Blankenship, R.E. Rapid one-step purification of the BChl-a containing FMO-protein from the green sulfur bacterium Chlorobium tepidum using a high efficiency immunomatrix. Photosynthesis Research 71, 149–154 (2002). https://doi.org/10.1023/A:1014907731595
Issue Date:
DOI: https://doi.org/10.1023/A:1014907731595