Abstract
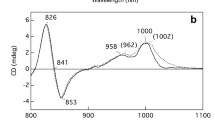
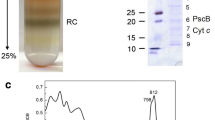
The BChla-containing Fenna-Matthews-Olson (FMO) protein from the green sulfur bacteriumChlorobium tepidum was purified and characterized. Fluorescence spectra indicate that efficient excited state quenching occurs at neutral or oxidizing redox potentials. The major fluorescence lifetime at room temperature is approximately 60 ps in samples that are in neutral or oxidizing conditions, and approximately 2 ns in samples where the strong reductant sodium dithionite has been added. A similar change is observed in pump-probe picosecond absorbance difference experiments, where the long life time component increases after dithionite addition. A 16 Gauss wide EPR signal with g factor =2.005 is observed in samples without dithionite. This signal largely disappears upon addition of dithionite. Dithionite induces large reversibile changes in the 77 K absorbance spectra of the purified FMO protein and in whole cells. These results indicate that the FMO protein contains redox active groups, which may be involved in the regulation of energy transfer. Room temperature circular dichroism and low temperature absorption spectra show that dithionite also induces conformational or structural changes of the FMO protein complex.
Similar content being viewed by others
Abbreviations
- BChl:
-
bacteriochlorophyll
- FMO protein:
-
Fenna-Matthews-Olson protein
References
Babcock GT, El-Deeb MK, Sandusky PO, Whittaker MM and Whittaker JW (1992) Electron paramagnetic resonance and electron nuclear double resonance spectroscopies of the radical site in galactose oxidase and of thioether-substituted phenol model compounds. J Am Chem Soc 114: 3727–3734
Barry BA and Babcock GT (1987) Tyrosine radicals are involved in the photosynthetic oxygen-evolving system. Proc Natl Acad Sci USA 84: 7099–7103
Blankenship RE (1985) Electron transport in green photosynthetic bacteria. Photosynth Res 6: 317–333
Blankenship RE, Cheng P, Causgrove TP, Brune DC, Wang SH, Choh JU and Wang J (1993) Redox regulation of energy transfer efficiency in antennas of green photosynthetic bacteria. Photochem Photobiol 57: 103–107
Blankenship RE, Wang J, Causgrove TP and Brune DC (1990) Efficiency and kinetics of energy transfer in chlorosome antennas from green photosynthetic bacteria. In: Baltscheffsky M (ed) Current Research in Photosynthesis, Vol II, pp 17–24. Kluwer Academic Publishers, Dordrecht, The Netherlands
Borg DC, Forman A and Fajer J (1976) ESR and ENDOR studies of the cation radical of bacteriochlorophyll. J Am Chem Soc 95: 6889–6893
Causgrove TP, Brune DC, Wang J, Wittmershaus BP and Blankenship RE (1990) Energy transfer kinetics in whole cells and isolated chlorosome of green photosynthetic bacteria. Photosynth Res 26: 39–48
Cheng P (1992) Characterization of energy transfer and structure of the antenna system of green photosynthetic bacteria. PhD dissertation. Arizona State University, Tempe, AZ
Daurat-Larroque ST, Brew K and Fenna RE (1986) The complete amino acid sequence of a bacteriochlorophylla-protein fromProsthecochloris aestuarii. J Biol Chem 261: 3607–3615
Davidson VL and Jones LH (1992) Cofactor-directed inactivation by nucleophilic amines of the quinoprotein methylamine dehydrogenase fromParacoccus denitrificans. Biochim Biophys Acta 1121: 104–110
Davis FS, Nemeth GA, Anjo DM, Makings LR, Gust D and Moor TA (1987) Digital back-off for computer controlled flash spectrometers. Rev Sci Inst 58: 1629–1631
Dracheva S, Williams JC and Blankenship RE (1992) Cloning and sequencing of the FMO-protein gene fromChlorobium tepidum. In: Murata N (ed) Research in Photosynthesis, Vol I, pp 53–56. Kluwer Academic Publishers, Dordrecht, The Netherlands
Johnson SG and Small GJ (1991) Excited-state structure and energytransfer dynamics of the bacteriochlorophylla antenna complex fromProsthecochloris aestuarii. J Phys Chem 95: 471–479
Karapetyan NV, Swarthoff T, Rijgersberg CP and Amesz J (1980) Fluorescence emission spectra of cells and subcellular preparations of a green photosynthetic bacterium, effects of dithionite on the intensity of the emission bands. Biochim Biophys Acta 593: 254–260
Kelly JM and Porter G (1970) The interaction of photo-excited chlorophylla with duroquinone, α-tocopherylquinone and vitamin K1. Proc R Soc London Ser A 319: 319–329
Knutson JR, Beeche JM and Brand L (1983) Simultaneous analysis of multiple fluorescence decay curves: A global approach. Chem Phys Lett 102: 501–507
Lin S, Chiou HC, Kleinherenbrink FAM and Blankenship RE (1994) Time-resolved spectroscopy of energy and electron transfer processes in the photosynthetic bacteriumHeliobacillus mobilis. Biophys J 66: 437–445
Lu XY and Pearlstein RM (1993) Simulations ofProsthecochloris bacteriochlorophylla-protein optical spectra improved by parametric computer search. Photochem Photobiol 57: 86–91
Matthews BW and Fenna R (1980) Structure of a green bacteriochlorophyll protein. Acc Chem Res 13: 309–317
Natarajan LV and Blankenship RE (1983) Free energy dependence of the quenching of chlorophylla fluorescence by substituted quinones. Photochem Photobiol 37: 329–336
Olson JM (1978) Bacteriochlorophylla-proteins from green bacteria. In: Clayton RK and Sistrom WR (eds) The Photosynthetic Bacteria, pp 161–178. Plenum, New York
Olson JM (1980) Bacteriochlorophylla-proteins of two green photosynthetic bacteria. Meth Enzymol 69: 336–344
Olson JM, Ke B and Thompson KH (1976) Exciton interaction among chlorophyll molecules in bacteriochlorophylla proteins and bacteriochlorophylla reaction center complexes from green bacteria. Biochim Biophys Acta 430: 524–537
Olson JM, Ormerod JG, Amesz J, Stackebrandt E and Trüper HG (eds) (1988) Green photosynthetic Bacteria. Plenum, New York
Olson JM, Philipson KD and Sauer K (1973) Circular dichroism and absorption spectra of bacteriochlorophyll-protein and reaction center complexes fromChlorobium thiosulfatophilum. Biochim Biophys Acta 292: 206–217
Orme-Johnson WH and Beinert H (1969) On the formation of the superoxide anion radical during the reaction of reduced ironsulfur proteins with oxygen. Biochem Biophys Res Comm 36: 905–9114
Paz MA, Fluckiger R, Boak A, Kagan HM and Gallop PM (1991) Specific detection of quinoproteins by redox-cycling staining. J Biol Chem 266: 689–692
Pearlstein RM (1992) Theory of the optical spectra of the bacteriochlorophylla antenna protein trimer fromProsthecochloris aestuarii. Photosynth Res 31: 213–226
Philipson KD and Sauer K (1972) Excitation interaction in a bacteriochlorophyll-protein from Chloropseudomonas ethylica. Absorption and circular Dichroism at 77 °K. Biochemistry 11: 1880–1885
Savikhin S, Zhou W, Blankenship RE and Struve WS (1994) Femtosecond energy transfer and spectral equilibration in FMO trimers from the green bacteriumChlorobium tepidum. Biophys J in press
Tronrud DE, Schmid MF and Matthews BW (1986) Structure and X-ray amino acid sequence of a bacteriochlorophylla protein fromProsthecochloris aestuarii refined at 1.9 Å resolution. J Mol Biol 188: 443–454
Tronrud DE and Matthews BW (1993) Refinement of the Structure of a Water-Soluble Antenna Complex from Green Photosynthetic Bacteria by Incorporation of the Chemically Determined Amino Acid Sequence. In: Norris J and Deisenhofer J (eds) The Photosynthetic Reaction Center, Vol 1, pp 13–21. Academic Press, San Diego, CA
VanGrondelle R (1985) Excitation energy transfer, trapping and annihilation in photosynthetic systems Biochim Biophys Acta 811: 147–195
VanMourik F, Verwijst RR, Mulder JM and VanGrondelle R (1993) Excitation transfer dynamics and spectroscopic properties of the light-harvesting BChla complex ofProsthecochloris aestuarii. J Lumin 53: 499–502
Van Mourik F (1993) Spectral inhomogeneity of the bacterial lightharvesting antennae: Causes and consequences. PhD thesis, Free University, Amsterdam, The Netherlands
Vos M, Nuijs AM, VanGrondelle R, VanDorssen RJ, Gerola PD and Amesz J (1987) Excitation transfer in chlorosomes of green photosynthetic bacteria. Biochim Biophys Acta 891: 275–285
Wang J, Brune DC and Blankenship RE (1990) Effects of oxidants and reductants on the efficiency of excitation transfer in green photosynthetic bacteria. Biochim Biophys Acta 1015: 457–463
Whittaker MM and Whittaker JW (1990) A tyrosine-derived free radical in apogalactose oxidase. J Biol Chem 265: 9610–9613
Whitten W, Olson JM and Pearlstein RM (1980) Seven-fold exciton splitting of the 810 nm band in bacteriochlorophylla-proteins from green photosynthetic bacteria. Biochim Biophys Acta 591: 203–207
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zhou, W., LoBrutto, R., Lin, S. et al. Redox effects on the bacteriochlorophyll α-containing Fenna-Matthews-Olson protein fromChlorobium tepidum . Photosynth Res 41, 89–96 (1994). https://doi.org/10.1007/BF02184148
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02184148