Abstract
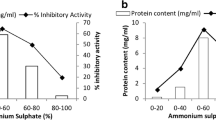
Plant protease inhibitors (PI) constitute a major class of defence proteins being selected as an important strategy towards insect herbivory. With the objective of assessing the effect of PI towards larval growth and development, an artificial diet bioassay using partially purified PI obtained from peas was performed on the melon fruit fly Bactrocera cucurbitae (Coquillett). Larval growth and developmental parameters were assessed at different concentrations (namely 12.5, 25, 50,100, 200 and 400 μg/ml) on the second-instar larvae of B. cucurbitae. Growth and survival responses determined the antiinsect potential of this PI even in its partially purified state. Larval and total development periods were found to be significantly prolonged for the larvae fed on an artificial diet incorporated with pea PI when compared with those fed with a control diet. Furthermore, when compared with the effect of the control diet (no inhibitor), the partially purified pea PI in the diet reduced larval weight gain, mean larval growth rate and food assimilated with respect to the control of the second-instar larvae tested at the same range of concentrations. The relative effectiveness of pea PI on these parameters is in agreement with the results obtained for percentage of pupation and percentage of adult emergence, as these parameters were significantly affected by the increase in the PI concentration in the artificial diet. Feeding the second-instar larvae a diet containing a range of concentrations (50,100,200 and 400 μg/ml) of partially purified pea PI significantly reduced the activities of digestive enzymes (trypsin, chymotrypsin, elastase and leucine aminopeptidase) and significantly affected the activities of other non-digestive enzymes (esterase, acid and alkaline phosphatases, glutathione S-transferase, superoxide dismutase and catalase).
Similar content being viewed by others
References
Applebaum S. W. (1986) Biochemistry of digestion, pp. 270–311. In Comprehensive Insect Physiology, Biochemistry and Pharmacology (edited by G. A. Kerkut and L. L. Gilbert). Vol. 4. Pergamon Press, New York, NY.
Bergmeyer H. U., Gawehn K. and Grassl M. (1974) Enzymes as biochemical reagents, pp. 425–522. In Methods of Enzymatic Analysis (edited by H. U. Bergmeyer). Academic Press, New York, NY.
Chaubey S. N. and Bhatt R. S. (1988) Changes in the levels of nucleic acid, protein, total free amino acid and glycogen, and activity of acid phosphatase in the eggs, during normal embryonic development of the rice moth, Corcyra cephalonica, Stainton (Lepidoptera: Pyralidae). Insect Biochemistry 18, 443–447.
Chien C. and Dauterman W. C. (1991) Studies on glutathione S-transferases in Helicoverpa (=Heliothis) zea. Insect Biochemistry 21, 857–864.
Christeller J. T., Laing W. A., Markwick N. P. and Burgess E. P. J. (1992) Midgut protease activities in 12 phytophagous lepidopteran larvae: dietary and protease inhibitor interactions. Insect Biochemistry and Molecular Biology 22, 735–746.
Christeller J. T., Laing W. A., Shaw B. D. and Burgess E. P. J. (1990) Characterization and partial purification of the digestive proteases of the black field cricket, Telleogryllus commodus (Walker): elastase is a major component. Insect Biochemistry 20, 157–164.
Clausen C. P., Clancy D. W. and Chock Q. C. (1965) Biological control of the oriental fruit fly (Dacus dorsalis Hendel) and other fruit flies in Hawaii. US Department of Agriculture Technical Bulletin 1322, 1–102.
Enayati A. A., Ranson H. and Hemingway J. (2005) Insect glutathione transferases and insecticide resistance. Insect Molecular Biology 14, 3–8.
Felton G. W. and Summers C. B. (1995) Antioxidant systems in insects. Archives of Insect Biochemistry and Physiology 29, 187–197.
Ferrason E., Quillien L. and Gueguen J. (1997) Proteinase inhibitors from pea seeds: purification and characterization. Journal of Agricultural Food Chemistry 45, 127–131.
Franco O. L., dos Santos R. C., Batista J. A. N., Mendes A. C. M., de Araujo M. A. M., Monnerat R. G., Grossi-de-Sá M. F. and de Freitas S. M. (2003) Effects of black-eyed pea trypsin/chymotrypsin inhibitor on proteolytic activity and on development of Anthonomus grandis. Phytochemistry 63, 343–349.
Gupta J. N., Verma A. N. and Kashyap R. K. (1978) An improved method for mass rearing for melon fruit fly Dacus cucurbitae Coquillett. Indian Journal of Entomology 40, 470–471.
Haq S. K., Atif S. M. and Khan R. H. (2004) Protein proteinase inhibitor genes in combat against insects, pests, and pathogens: natural and engineered phytoprotection. Archives of Biochemistry and Biophysics 431, 145–159.
Haq I., Caceres C., Hendrichs J., Teal P. E. A., Wornoayporn V., Stauffer C. and Robinson A. S. (2010a) Effects of the juvenile hormone analogue methoprene and dietary protein on male melon fly Bactrocera cucurbitae (Diptera: Tephritidae) mating success. Journal of Insect Physiology 56, 1503–1509.
Haq I., Mayr L., Teal P. E. A., Hendrichs J., Robinson A. S., Stauffer C. and Hood-Nowotny R. (2010b) Total body nitrogen and total body carbon as indicators of body protein and body lipids in the melon fly Bactrocera cucurbitae: effects of methoprene, a juvenile hormone analogue, and of diet supplementation with hydrolysed yeast. Journal of Insect Physiology 56, 1807–1815.
Jongsma M. A. and Bolter C. (1997) The adaptation of insects to plant protease inhibitors. Journal of Insect Physiology 43, 885–895.
Katzenellenbogen B. and Kafatos F. C. (1971) General esterases of silkmoth moulting fluid: preliminary characterization. Journal of Insect Physiology 17, 1139–1151.
Khan Z. R. and Saxena R. C. (1985) Behavioral and physiological responses of Sogatella furcifera (Homoptera: Delphacidae) to selected resistant and susceptible rice cultivars. Journal of Economic Entomology 78, 1280–1286.
Koiwa H., Bressan R. A. and Hasegawa P. M. (1997) Regulation of protease inhibitors and plant defense. Trends in Plant Sciences 2, 379–384.
Kono Y. (1978) Generation of superoxide radical during auto-oxidation of hydroxylamine and an assay for superoxide dismutase. Archives of Biochemistry and Biophysics 186, 189–195.
Koyama T., Kakinohana H. and Miyatake T. (2004) Eradication of the melon fly Bactrocera cucurbitae in Japan: importance of behaviour, ecology, genetics and evolution. Annual Review of Entomology 49, 331–349.
Lowry O. H., Rosebrough A., Lewis F. A. and Randall R. J. (1951) Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry 193, 263–275.
Ma C. C. and Kanost M. R. (2000) A beta 1,3-glucan recognition protein from an insect, Manduca sexta agglutinates microorganisms and activates the phenoloxidase cascade. Journal of Biological Chemistry 275, 7505–7514.
Martinez S. S. and van Emden H. F. (2001) Growth distribution, abnormalities and mortality of Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae) caused by azadirachtin. Neotropical Entomology 30, 113–125.
McIntyre R. J. (1971) A method for measuring activities of acid phosphatases separated by acrylamide gel electrophoresis. Biochemical Genetics 5, 45–50.
Nanasahe P. C., Doyle E., Fitches E. and Gatehouse J. A. (2008) Biochemical characterization of midgut digestive proteases from Mamestra brassicae (cabbage moth; Lepidoptera: Noctuidae) and effect of soybean Kunitz inhibitor (SKTI) in feeding assays. Journal of Insect Physiology 54, 563–572.
Oppenorth F. J. (1985) Biochemistry and genetics of insecticide resistance, pp. 731–773. In Comprehensive Insect Physiology, Biochemistry and Pharmacology (edited by G. A. Kerkut and L. I. Gilbert). Vol. 12. Pergamon Press, Oxford.
Paes M. C., Oliveria M. B. and Oliveria P. L. (2001) Hydrogen peroxide detoxification in the midgut of the blood-sucking insect, Rhodnius prolixus. Archives of Insect Biochemistry and Physiology 48, 63–71.
Paulino da Silva L., Leite J. R. S. A., Bloch C. Jr and Maria de Freitas S. (2001) Stability of a black eyed pea trypsin/chymotrypsin inhibitor (BTCI). Protein and Peptide Letters 8, 33–38.
Rahbé Y., Ferrasson E., Rabesona H. and Quillien L. (2003) Toxicity to the pea aphid Acyrthosiphon pisum of antichymotrypsin isoforms and fragments of Bowman-Birk protease inhibitors from pea seeds. Insect Biochemistry and Molecular Biology 33, 299–306.
Reeck G. R., Oppert B., Denton M., Kanost M., Baker J. E. and Kramer K. J. (1999) Insect proteinases, pp. 125–148. In Proteases: New Perspectives (edited by V. Turk). Birkauzer Verlag, Boston.
Rockstein M. (1956) Phosphatases of house fly, Musca domestica (L.). Bulletin of Brooklyn Entomological Society 51, 9–17.
Roessler Y. (1989) Control; insecticides; insecticidal bait and cover sprays, pp. 329–336. In World Crop Pests 3(B). Fruit Flies; Their Biology, Natural Enemies and Control (edited by A. S. Robinson and G. Hooper). Elsevier, Amsterdam.
Ryan C. A. and Pearce G. (1998) Systemin: a polypeptide signal for plant defensive genes. Annual Review of Cell and Developmental Biology 14, 1–17.
Srivastava B. G. (1975) A chemically defined diet for Dacus cucurbitae (Coq.) larvae under aseptic conditions. Entomology News Letter 5, 24.
Terra W. R. and Ferreira C. (1994) Insect digestive enzymes: properties, compartmentalization and function. Comparative Biochemistry and Physiology B 109, 1–62.
Wang X. (2001) The expanding role of mitochondria in apoptosis. Genes and Development 15, 2922–2933.
White I. M. and Elson-Harris M. M. (eds) (1992) Fruit Flies of Economic Significance: Their Identification and Bionomics. CAB International, Wallingford. 601 pp.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaur, A.P., Sohal, S.K. Biopotency of partially purified protease inhibitor from peas on the larval growth, development and enzyme system of Bactrocera cucurbitae (Diptera: Tephritidae). Int J Trop Insect Sci 33, 82–90 (2013). https://doi.org/10.1017/S1742758412000446
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1017/S1742758412000446