Abstract
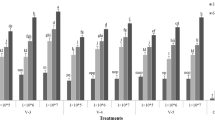
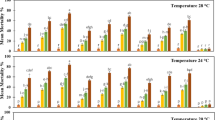
Isolates of Beauveria bassiana (Balsamo) Vuillemin, Paecilomyces farinosus (Holm ex SF Gray) Brown and Smith and Verticillium lecanii (Zimmermann) Viegas were evaluated for their pathogenicity to adults, eggs, first and third instars of silverleaf whitefly, Bemisia argentifolli (Bellows and Perring) (Homoptera: Aleyrodidae). Conidial dilutions were prepared in 0.05% Tween® and leaves treated by immersion in these suspensions. Assessments of nymphal infection were made 2, 3, 5, 7, and 10 days post-treatment. Infection rates in adults and eggs were made 14 days post-treatment. All isolates were pathogenic to nymphs and adults. None of the isolates were pathogenic to eggs. Isolates L3444 (P. farinosus) and L3009 (B. bassiana) were the most pathogenic to first instars and adults with LC50 and LC90 values of 7.3 × 104 and 3.1 × 106 conidia/ml, and 1.3 × 106 and 1.0 × 107 conidia/ml, respectively. Isolate FR20 (V. lecanii) was the most pathogenic to third instars with LC50 and LC90 values of 4.8 × 103 and 7.4 × 105 conidia/ml, respectively. Results imply that L3444 and L3009 are the most efficient isolates, based on their pathogenicity to first instars and adults. Results are discussed in relation to using fungi for management of silverleaf whitefly in greenhouses.
Résumé
Des isolats de Beauveria bassiana ( Balsamo) Vuillemin, Paecillomyces farinosus (Holm ex SF) Brown et Smith, et Verticillium lecanii (Zimmerman) Viegas ont été évalués quant à leur pathogénicité pour les adultes, les oeufs et les larves du premier et troisième stades de développement de l’aleurode du peuplier, Bemisia argentifolli (Bellows et Perring) (Homoptera: Aleyrodidae). Les dilutions de conidies étaient préparées dans une solution du Tween 0,05%® et les feuilles traitées par immersion dans ces suspensions. La recherche des nymphes infectées fut effectuée 2,3,5, 7 et 10 jours après le traitement. Le taux d’infection chez les adultes et les oeufs était évalué 14 jours après le traitement. Tous les isolats ont montré un pouvoir pathogène vis-à-vis des nymphes et les adultes mais aucun d’entre eux n’a manifesté de pathogénicité à l’égard des oeufs. Les isolats L3444 (P. farinosus) et L3009 (B. bassiana) étaient les plus pathogènes vis-à-vis des premiers stades de développement et des adultes. A la CL50 et CL90, les concentrations respectives étaient de 7,3 × 104 et de 3,1 × 106 conidies / ml pour les premiers stades, et de 1,3 × 106 et de 1,0 × 107 conidies / ml pour les adultes. L’isolat FR20 (V. lecanii) fut le plus effectif pour nymphes du 3ème stade de développement avec des valeurs respectives de la CL50 et CL90 équivalentes à 4,8 × 103 et 7,4 × 105 conidies / ml. Les résultats suggèrent que, compte tenu de leur pathogénicité vis-à-vis des premiers stades et des adultes, les isolats L3444 et L3009 sont les plus efficaces. Les résultats discutent aussi de l’utilisation des produits fongiques pour le contrôle de l’aleurode du peuplier argenté dans les serres.
Similar content being viewed by others
References
Aguda R. M. and Rombach M. C. (1986) Handling of insect fungi, pp. 117–123. In Proceedings of the Southeast Asian Regional Training Course on Microbial Control of Insect Pests and Plant Diseases in the Tropics. National Institute of Biotechnology and Applied Microbiology (BIOTECH), U. P. Los Baños, Laguna, Philippines. October 19–26, 1986. University of Philippines, Los Baños.
Brownbridge M., Parker B. L. and Skinner M. (1992) Development of insect-killing fungi for greenhouse use: A more sustainable pest management approach, pp. 39–40. In Sustaining a Vermont Way of Life: Research and Education in Sustainable Agriculture at the University of Vermont, Plant and Soil Science Department and Extension System (Edited by E. Seyler with assistance from F. Magdoff, K. Duesterburg, D. Heleba, B. Holtzman and B. Fardleman). University of Vermont.
Drummond J. and Heale J. B. (1988) Genetic studies on the inheritance of pathogenicity in Verticillium lecanii against Trialeurodes vaporariorum. J. Invertebr. Pathol. 52, 57–65.
Drummond J., Heale J. B. and Gillespie A. T. (1987) Germination and effect of reduced humidity on the expression of pathogenicity in Verticillium lecanii against the greenhouse whitefly, Trialeurodes vaporariorum. Ann. Appl. Biol. 111, 193–201.
Ekbom B. S. (1979) Investigation on the potential of parasitic fungus (Verticülium lecanii) for biological control of greenhouse whitefly (Trialeurodes vaporariorum). Swedish J. Agric. Res. 9, 129–138.
Ekbom B. S. and Ahman I. (1980) The fungus Verticillium fusisporum as an insect pathogen. J. Invertebr. Pathol. 36, 136–138.
Feng M. G. and Johnson J. B. (1990) Relative virulence of six isolates of Beauveria bassiana on Diuraphis noxia (Homoptera: Aphididae). Environ. Entomol. 19, 785–790.
Fransen J. J., Winkelmann C. and van Lenteren J. C. (1987) The different mortality at various stages of the greenhouse whitefly, Trialeurodes vaporariorum (Homoptera: Aleyrodidae), by infection with the fungus Ashersonia aleyrodis (Deuteromycotina: Coelomycetes). J. Invertebr. Pathol. 50, 158–165.
Hall R. A. (1976) A bioassay of the pathogenicity of Verticillium lecanii conidiospores on the aphid, Macrosiphoniella sanborni. J. Invertebr. Pathol. 27, 41–48.
Hall R. A. (1985) Whitefly control by fungi, pp. 116–118. In Biological Pest Control—The Glasshouse Experience (Edited by N. W. Hussey and N. Scopes). Cornell Univ. Press, Ithaca, NY.
Herakly F. A. and El-Ezz A. A. (1970) The cotton whitefly, Bemisia tabad Genn., infesting cucurbits, in U. A. R. Agric. Res. Rev. 48, 110–118.
Hussey N. W. (1985) History of biological control in protected culture, Western Europe, pp. 11–22. In Biological Pest Control—The Glasshouse Experience (Edited by N. W. Hussey and N. Scopes). Cornell Univ. Press, Ithaca, NY.
Kanagaratnam P., Hall R. A. and Burges H. D. (1982) Control of glasshouse whitefly, Trialeurodes vaporariorum, by an aphid isolate of the fungus Verticillium lecanii. Ann. Appl. Biol. 100, 213–219.
Lopez-Avila A. and Cock M. J. W. (1986) Economic damage, pp. 51–54. In Bemisia tabad—A Literature Survey on the Cotton Whitefly with Annotated Bibliography (Edited by M. J. Cock). CAB International Institute of Biological Control, Silwood Park, Ascot, Berks SL5 7PY UK.
Matthews G. A. (1986) Overview of chemical control with spedal reference to cotton crops, pp. 51–54. In Bemisia tabaci—A Literature Survey on the Cotton Whitefly with Annotated Bibliography (Edited by M. J. Cock). CAB International Institute of Biological Control, Silwood Park, Ascot, Berks SL5 7PY UK.
McHugh J. B. (1991) Yes, you can win the war on whiteflies. Greenhouse Grower, Spring, 1–2.
Ondieki J. J. (1975) Diseases and pests of passion fruit in Kenya. Acta Horticulturae 49, 291–293.
Osborne L. S. and Landa Z. (1992) Biological control of whitefly with entomopathogenic fungi. Florida Entomol. 75, 456–471.
Reinecke P. (1990) Biological control products: Demands of industry for successful development, pp. 99–108. In Pesticides and Alternatives: Innovative Chemical and Biological Approaches to Pest Control (Edited by J. E. Casida). Elsevier Science Publisher. Amsterdam, Oxford, NY.
Sanderson J. P. and Ferrentino G. W. (1991) Whiteflies 101: A primer on biology and control. Grower Talks 55, 48–61.
SAS Institute (1987) SAS User’s Guide: Statistics. SAS Institute, Cary, NC.
Shaheen A. H. (1977) Survey of pests attacking tomato in Egypt with some ecological notes. Agric. Res. Rev. 55, 49–57.
van der Schaaf D. A., Ravensberg W. J. and Malais M. (1991) Verticullum lecanii as a microbial insectidde against whitefly, pp. 120–123. In Proc. Third European Meeting on “Microbial Control of Pests” (Edited by P. H. Smits). Wageningen, The Netherlands, 24–27 February 1991. IOBC/WPRS Bulletin XIV/7.
van Lenteren J. C. and Noldus L. P. J. (1990) Whitefly-plant relationships: Behavioral and ecological aspects. In Whiteflies: Their Bionomics, Pest Status and Management, pp. 47–89 (Edited by D. Gerling). Intercept Ltd., Andover, Hants, UK.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Negasi, A., Parker, B.L. & Brownbridge, M. Screening and Bioassay of Entomopathogenic Fungi for the Control of Silverleaf Whitefly, Bemisia argentifolli. Int J Trop Insect Sci 18, 37–44 (1998). https://doi.org/10.1017/S174275840000744X
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1017/S174275840000744X