Abstract
The whitefly, Bemisia tabaci (Genn.) (Hemiptera: Aleyrodidae), is one of the most damaging pests in field crops as well as in greenhouses. The present study aimed to evaluate the in vitro pathogenicity of 2 fungus strains of Beauveria bassiana (BB-72 and BB-252) and one strain of Lecanicillium lecanii (V-2) against the whitefly (B. tabaci) at 4 different temperature degrees 20, 24, 28, and 32 °C, using a spray method. Three different bioassays were carried out comprised of conidial concentrations and filtrate of fungal strains, BB-72, BB-252 and V-2, and their binary combinations. The 1.5 ml of fungal filtrate was used in filtrate bioassay for each strain of fungus. Three different concentrations (1 × 106, 1 × 107, and 1 × 108 conidia ml−1) were used in conidial bioassay for each strain of fungus, whereas in bioassay for binary combination (1 ml conidia + 1 ml filtrate) of BB-72 × BB-72, BB-252 × BB-252, and V2 × V2 were used for these strains of fungus. According to the outcomes, the maximum mortality against whiteflies was observed on 12th day post-treatment. In conidial bioassay, the maximum mortality of B. tabaci was observed in BB-72 isolate (84%), BB-252 isolate (77%), and V-2 isolate (67%) at the highest concentration (1 × 108 conidia ml−1) at 24 °C, and minimum mortality was recorded in BB-72 isolate (33%), BB-252 isolate (29%), and V-2 isolate (19%) at the lowest concentration 1 × 106 conidia ml−1 at 32 °C on 12th day post-treatment. Infiltrate bioassay, BB-72 isolate exhibited maximum mortality (92%) at 24 °C, and V-2 isolate showed minimum mortality (34%) at 32 °C on 12th day post-treatment. Furthermore, in binary combination bioassay, the highest whitefly mortality was recorded in BB-72 × BB-72 isolate (87%), BB-252 × BB-252 isolate (73%), and V2 × V2 isolate (65%) at 24 °C and the lowest mortality in BB-72 × BB-72 isolate (57%), BB-252 × BB-252 isolate (50%), and V2 × V2 isolate (39%) at 32 °C on 12th day post-treatment. In all bioassays, the BB-72 isolate was the utmost virulent, and application of its filtrate was found to be the most impressive against B. tabaci.
Similar content being viewed by others
Background
The whitefly, Bemisia tabaci (Genn.) (Hemiptera: Aleyrodidae), is one of the most destructive pest in the field crops as well as in greenhouses. It directly damages the crops causing heavy losses of the crops (Oliveira et al. 2001). Entomopathogenic fungi (EPF) have the ability to individually affect their host directly through the inward penetration in an insect (Lacey et al. 1996). Beauveria bassiana, V. lecanii, and Metarhizium anisopliae proved to be the most promising fungi in this respect (Wraight et al. 2007). For the control of whitefly, the entomopathogenic fungi have been used almost all around the world (Jackson et al. 2010). The potential strains best for utilization as pest management tools can be categorized into two groups: one group includes Hyphomycetes in Deuteromycotina and the other belongs to Entomophthorales (EPF) in Zygomycota (Feng 1998).
Very few of these EPF, viz., Nomuraea rileyi, Beauveria bassiana, Paecilomyces fumosoroseus, Metarhizium anisopliae, B. brongniartii, and Verticillium lecanii have been registered as products to control insect pests (Shah and Goettel 1999). B. tabaci showed resistance against the chemical insecticides such as nitenpyram, imidacloprid, acetamiprid, and pyrethroid (Horowitz et al. 2008). In china, many recent studies have revealed that most of the commonly available pesticides have lost their effectiveness in the control of whitefly (Wang et al. 2009). Therefore, the development of alternative sources of control is urgently needed. Biological control has now become key area of research in the management of B. tabaci over the past 2 decades (Cuthbertson et al. 2010). A number of microbial bio-pesticides based on entomopathogenic organisms like bacteria, nematodes, viruses, and fungi have been playing key roles in plant protection and were used against a broad range of insect pests (Majeed MZ et al. 2017). Entomopathogenic fungi like M. anisopliae, Isaria fumosorosea, B. bassiana, and L. lecanii proved to be impressive, target-specific, and environment-friendly biocontrol agents against several species of sucking insect pests (Cabanillas and Jones 2009). After germination of spores connected to the target insect’s cuticle, fungal hyphae enter the body of the insect and cause death of host within a day (Mora et al. 2017). In addition, EPF have less or no residual activity or mammalian toxicity and are specific to the target pest, as well as there is less risk for developing resistance (Zimmermann 2007).
B. bassiana and L. Lecanii are the most studied EPF and known as a virulent biocontrol agent of a wide range of forest, field crops, and green house pests. These entomopathogens were equally effective in desert, agricultural, and forest habitats (Annamalai et al. 2016 and Dogan et al. 2017). Lately, (Nazir et al. 2019) demonstrated the toxicity of filtrates and conidia derived from different isolates of L. lecanii and B. bassiana against the aphid species, Myzus persicae (Sulz.).
The strains of both the entomopathogens can be easily isolated from the infected soil dwelling pests or from vegetation pests (Freed et al. 2011). As with other organisms, the growth rate of EPF varies by temperature. They are usually cultured nearby 25 °C, even depending on the strain in a single species (Sato 1993). The aim of the present study was to assess the pathogenicity and virulence of EPF, i.e., L. lecanii and B. bassiana against B. tabaci under controlled environmental conditions.
Materials and methods
Whitefly stock culture
Bemisia tabaci individuals were collected from the seedlings of tomato (Solanum lycopersicum) grown in the greenhouse at Chinese Academy of Agricultural Sciences (CAAS) Beijing, China. The population of whitefly was reared on the seedlings of same tomato grown in pots kept in cages at 25 ± 2 °C and 50 60% RH with photoperiod of 16:8 h light:dark (L:D). In the entire study period, the plants were replaced weekly with new ones.
Effect of temperature on fungus virulence against Bemisia tabaci
Evaluation of the role of temperature, the pathogenicity bioassays of B. bassiana and L. lecanii against the nymphs of whitely, was carried out at 20, 24, 28, and 32 °C.
Fungal isolates
Two isolates of B. bassiana, namely, BB-72 and BB-252, and one isolate of L. lecanii, V-2 (Table 1), were cultured on potato dextrose agar (PDA) medium (agar 20 g/l., dextrose 20 g/l, and potatoes 200 g/l) plates and incubated at 24 ± 2 °C in dark for 20–25 days.
Conidial suspensions
The conidia were harvested from 25 days of fungal culture plates using ddH2O with 0.01% Tween 80® (Sigma-Aldrich, Saint Louis, MO, USA). The suspensions of conidia were collected and then filtered by Miracloth (EMD Milipore Corp., Billerica, MA, USA). Neubauer hemocytometer (Brand GmbH, Wertheim, Germany) was used to adjust the conidial concentrations at 1 × 106, 1 × 107, and 1 × 108 conidia ml−1. Before doing bioassays against whitefly, the conidial viability test was carried out, according to the methods described by (Hywel-Jones and Gillespie 1990).
Fungal filtrate
Adamek’s liquid medium (40 g yeast extract (Difco, Detroit, MI, USA), 40 g dextrose and 30 g corn steep liquor (Sigma-Aldrich, Saint Louis, MO, USA), was used for primary culture of fungal filtrate by adding 4 ml of conidial suspension into 100 ml of medium for all the strains of fungi. Cultures were incubated for 72 h at 150 rpm, and then for secondary culture, 5 ml from primary culture was added into 500 ml of Adamek’s liquid medium and incubated at 26 °C and 150 rpm for 6 days for each strain. The samples were then centrifuged at 12000 rpm and 4 °C for 30 min. After that, the supernatant was filtered through 0.45 μm pore size filter (Millipore Corp., Billerica, MA, USA) to get filtrate, and pH was sustained at 6.
Pathogenicity bioassays
To evaluate the efficacy of filtrate, conidia, and binary combinations, different bioassays were carried out. For binary combination bioassays (conidia + filtrate), 1 ml of the highest concentration of conidia 1 × 108 conidia ml−1 of each fungal isolate was combined with 1 ml filtrate of the same fungal isolate. The combinations were BB-72 × BB-72, BB-252 × BB- 252, and V2 × V2. For all treatments ,the 90-mm Petri dishes were used with a skinny layer of 1.5% agar, and 50 mm diameter of leaf discs of tomato was placed in Petri dishes. In the control treatment, the leaf discs were sprayed only by 0.01% Tween 80®. Ten apterous adult of B. tabaci were released on the leaf discs at each Petri dish, and 1.5 ml sample of each of the treatments was sprayed on B. tabaci with the help of small sprayer and then these Petri dishes were incubated at 20, 24, 28, and 32 °C and 50–60% RH with photoperiod of 16:8 h light:dark (L:D) for 12 days. Ten replications of each treatment were used. B. tabaci mortality was recorded after 3, 6, 9, and 12 days post-treatment.
Data analysis
Virulence of the two strains of B. bassiana and one strain of L. lecanii as filtrate, conidia, and binary combination (conidia + filtrate) were analyzed, using factorial analysis of the variance (Statistix software (version 8.1) (Tallahassee, FL)). Comparisons of the treatment means were performed using Fischer’s least significant difference (LSD) test at a = 0.05.
Result and discussion
Conidial virulence
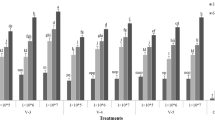
In conidial bioassay, the results showed that all the isolates tested revealed significant mortality of whitefly individuals (F = 101.30, p < 0.001; Table 2). Moreover, the factorial analysis of variance exhibited that there was a significant effect of temperature levels, time intervals, concentrations, and their interaction on whitefly mortality rate (Table 2). The maximum mortality of B. tabacii was observed in BB-72 isolate (84%), BB-252 isolate (77%), and V-2 isolate (67%) at the highest concentration 1 × 108 conidia ml−1, and low mortalities were recorded at the lowest concentration 1 × 106 conidia ml−1 on 12th day of post-treatment at 24 °C (Fig. 1). The minimum mortality of whitefly was recorded by BB-72 isolate (33%), BB-252 isolate (29%), and V-2 isolate (19%) at the lowest concentration 1 × 106 conidia ml−1 on 12th day of post-treatment at 32 °C (Fig. 1). The mean mortality at the highest concentration 1 × 108 conidia ml−1 in BB-72 isolate (74%), BB-252 isolate (68%), and V-2 isolate (61%,) and at the lowest concentration 1 × 106 conidia ml−1 in BB-72 isolate (46%), BB-252 isolate (40%), and V-2 isolate (33%) was recorded on 12th day of post-treatment at 28 °C (Fig. 2). The mean mortality was at the lowest concentration 1 × 106 conidia ml−1in BB-72 isolate (36%), BB-252 isolate (33%), and V-2 isolate (23%), and at the highest concentration 1 × 108 conidia ml−1 in BB-72 isolate (59%), BB-252 isolate (52%), and V-2 isolate (44%) was recorded on 12th day of post-treatment at 20 °C (Fig. 1). The mean mortality of B. tabaci in the control Petri-plates was noticeably low (6%) after 12th day post-treatment.
Mean mortality percentage of Bemisia tabaci recorded at different temperatures, concentrations, and time intervals (DAT: days after treatment) for conidia bioassays carried out with two strains of Beauveria bassiana (BB-72 and BB-252) and one strain of Lecanicillium lecanii (V-2). Columns represent mean percent mortality ± SE (n = 10). Treatment columns bearing different alphabets are significantly different from other treatments (least significant difference (LSD) test at α = 0.05)
Mean mortality percentage of Bemisia tabaci recorded at different temperature and different time intervals (DAT: days after treatment) for filtrate bioassays carried out with two strains of Beauveria bassiana (BB-72 and BB-252) and one strain of Lecanicillium lecanii (V-2). Columns represent mean percent mortality ± SE (n = 10). Treatment columns bearing different alphabets were significantly from other treatments (least significant difference (LSD) test at α = 0.05)
Filtrate pathogenicity
Results of the filtrate bioassay showed that the mortality percentages of whitefly by all isolates of the fungus were more than those of the conidial treatments. The fungal filtrate, observation time, temperature, and their interactions had significant effects on B. tabaci mortality (Table 3). Maximum mortality of B. tabaci was observed in BB-72 isolate (92%), BB-252 isolate (83%), and V-2 isolate (72%) on 12th day of treatment at 24 °C. Minimum mortality was observed in BB-72 isolate (55%), BB-252 isolate (43%), and V-2 isolate (34%) on 12th day of treatment at 32 °C. On the other hand, the mean mortality of BB-72 isolate (76%), BB-252 isolate (64%), and V-2 isolate (56%) was on 12th day of treatment at 28 °C, while the mean mortality of BB-72 isolate (61%), BB-252 isolate (53%), and V-2 isolate (45%) was on 12th day of treatment at 20 °C (Fig. 2).
Binary combinations
The bioassay was carried out with the binary combinations of (conidia + filtrate) of two strains of B. bassiana (BB-72 and BB-252) and one strain of L. lecanii (V-2), and they showed significant control of B. tabaci. On B. tabaci mortality, the binary combinations (conidia + filtrate), observation time, temperature, and their interactions had significant effects (Table 4). Significant and the highest white fly mortality was recorded in BB-12th day of treatment at 24 °C, while the lowest white fly mortality was recorded in BB-72 × BB-72 isolate (57%), BB-252 × BB-252 isolate (50%), and V2 × V2 isolate (39%) on 12th day of treatment at 32 °C. The mean mortality of BB-72 × BB-72 isolate (74%), BB-252 × BB-252 isolate (60%), and V2 × V2 isolate (51%) on 12th day of treatment at 28 °C, whereas the mean mortality of BB-72 × BB-72 isolate (64%), BB-252 × BB-252 isolate (50%), and V2 × V2 isolate (50%) on 12th day of treatment at 20 °C (Fig. 3).
Mean mortality percentage of Bemisia tabaci recorded at different temperature and time intervals (DAT: days after treatment) for binary combinations (conidia + filtrate) and bioassays carried out with two strains of Beauveria bassiana (BB-72 × BB-72 and BB-252 × BB-252) and one strain of Lecanicillium lecanii (V2 × V2). Columns represent mean percent mortality ± SE (n = 10). Treatment columns bearing different alphabets are significantly different from other treatments (least significant difference (LSD) test at α = 0.05)
The development of biocontrol research tools based on entomopathogenic fungi with improved efficacy against the target pests is one of the key areas of biological control research. Several insect pests have been controlled by the different entomopathogenic fungal strains (Yun et al. 2017).
Obtained results are in accordance with the previous studies describing the effectiveness of different isolates of L. lecanii and B. bassiana against sucking insect pests (Hesketh et al. 2008). In all bioassays, the mortality of whiteflies was dependent on temperature, time, and concentration, and it increased by increasing the concentrations of conidia and time after application at optimum temperature. Mortality of 92, 83 and 72% was recorded after 12 days of filtrate application of BB-72, BB-252, and V-2 isolate, in that order, while 84, 77, and 67% mortality of whiteflies was found after 12 days at the highest concentration 1 × 108 conidia ml−1 of BB-72, BB-252, and V-2 isolate at 24 °C. Another experiment was carried out and reported 87, 73, and 65% mortality of whiteflies after 12 days through binary combination of conidia + filtrate of BB-72 × BB-72, BB-252 × BB-252, and V2 × V2 isolates. These results are in accordance with (Wraight et al. 2000) who reported that B. bassiana caused up to 97% mortality, and V. lecanii showed 100% mortality of Chilo partellus. Obtained results are in accordance with (Halimona and Jankevica 2011), who tested various concentrations of B. bassiana against A. fabae and Metopeurum fuscoviride and found that the highest concentration of conidia 1 × 108 ml−1 showed maximum mortality after 7th day of application.
The percentage of mortality was affected by temperature, conidial concentration, and exposure time (Ansari et al. 2004). B. bassiana showed a great effect on the B. tabaci and Aphis craccivora infesting cucumber (Maniania 1991). The V. lecanii showed high mortality in early stages of B. tabaci and less mortality in old instars (Zaki 1998). EPF showed a good control of B. tabaci (Abdel-Raheem et al. 2016). The filtrate application was more effective to control the insect pests than the conidial application mainly in the case of short life cycled insects. There may be a possible cause that the less attachment of conidia to the insect cuticle as compared to huge infiltration of filtrates (Hanan et al. 2020ab).
Concerning the combined application obtained results is identical to (Yun et al. 2017) who studied the double behavior of EPF B. bassiana and M. anisopliae against M. persicae and B. cinerea and witnessed that the usage of filtrate with blastospores provided the maximum mortality of M. persicae. Furthermore, the mortality percentage was near to the combination of filtrates with blastospores; however, the combination of conidia and filtrate gave the minimum mortality as compared to filtrate application. So, it is clear that the fungal filtrate application had extreme virulence efficiency. Moreover, EPF are considered safe and environmentally friendly than the chemical pesticides (Goettel and Jaronski 1997); so, they are suggested as a control agent against destructive insect pests, like whitefly B. tabaci.
Conclusion
The in vitro study exhibited the efficiency of 2 strains of B. bassiana and one strain of L. lecanii against B. tabaci. L. lecanii showed lower mortality than both strains of B. bassiana either singly or in binary combinations (conidia + filtrate) of same strain at optimum temperature. The application of filtrate was utmost appropriate material to control B. tabaci. The binary combination of filtrate + its conidia had some irreconcilable effect and cannot effectively be used to control B. tabaci. Further studies are still needed to assess the role of EPF against B. tabaci, especially under field conditions.
Availability of data and materials
All data and materials are mentioned in the manuscript.
Abbreviations
- PDA:
-
Potato dextrose agar
- ddH2o:
-
Double-distilled water
- L:D:
-
Light:dark
- DF:
-
Degree of freedom
- μm:
-
Micrometer
- ml:
-
Milliliter
- pH:
-
Potential of hydrogen
- Rpm:
-
Revolutions per minute
- DAT:
-
Days after treatment
- SS:
-
Sum of squares
- MS:
-
Mean sum of squares
- F:
-
F-statistic
- CV:
-
Coefficient of variation
- GM:
-
Grand mean
References
Abdel-Raheem MA, Reyad NF, Abdel-Rahman IE, Al-Shuraym L (2016) Evaluation of some isolates of entomopathogenic fungi on some insect pests infesting potato crop in Egypt. Int J Chem Tech Res 9:479–485
Annamalai M, Kaushik HD, Selvaraj K (2016) Bioefficacy of Beauveria bassiana (Balsamo) Vuillemin and Lecanicillium lecanii Zimmerman against Thrips tabaci Lindeman. Proc Natl Acad Sci India Sect B Biol Sci 86:505–511
Ansari MA, Vestergaard S, Tirry L, Moens M (2004) Selection of a highly virulent fungal isolate, Metarhizium anisopliae CLO 53, for controlling Hoplia philanthus. J Invertebr Pathol 85:89–96
Cabanillas HE, Jones WA (2009) Pathogenicity of Isaria sp.(Hypocreales: Clavicipitaceae) against the sweet potato whitefly B biotype, Bemisia tabaci (Hemiptera: Aleyrodidae). Crop Prot 28:333–337
Cuthbertson AGS, Blackburn LF, Northing P et al (2010) Chemical compatibility testing of the entomopathogenic fungus Lecanicillium muscarium to control Bemisia tabaci in glasshouse environment. Int J Environ Sci Technol 7:405–409
Dogan YO, Hazir S, Yildiz A et al (2017) Evaluation of entomopathogenic fungi for the control of Tetranychus urticae (Acari: Tetranychidae) and the effect of Metarhizium brunneum on the predatory mites (Acari: Phytoseiidae). Biol Control 111:6–12
Feng MG (1998) Diversity of entomopathogenic fungi as resources useful for microbial control of insect pests. In: Proceedings of the First International Symposium on the Geoenvironmental Changes and Biodiversity in the Northeast Asia. pp 16–19
Freed S, Jin F-L, Ren S-X (2011) Phylogenetics of entomopathogenic fungi isolated from the soils of different ecosystems. Pakistan J Zool 43:417–425
Goettel MS, Jaronski ST (1997) Safety and registration of microbial agents for control of grasshoppers and locusts. Mem Entomol Soc Canada 129:83–99
Halimona J, Jankevica L (2011) The influence of Entomophthorales isolates on aphids Aphis fabae and Metopeurum fuscoviride. Latv Entomol 50:55–60
Hanan A, Basit A, Nazir T, Majeed MZ, Qiu D (2020b) Anti-insect activity of a partially purified protein derived from the entomopathogenic fungus Lecanicillium lecanii (Zimmermann) and its putative role in a tomato defense mechanism against green peach aphid. J Inv Pathol 170:107282
Hanan A, Nazir T, Basit A et al (2020a) Potential of Lecanicillium lecanii (Zimm.) as a microbial control agent for green peach aphid, Myzus persicae (Sulzer)(Hemiptera: Aphididae). Pak J Zool 52
Hesketh H, Alderson PG, Pye BJ, Pell JK (2008) The development and multiple uses of a standardised bioassay method to select hypocrealean fungi for biological control of aphids. Biol Control 46:242–255
Horowitz R, Kontsedalov S, Khasdan V et al (2008) The biotypes B and Q of Bemisia tabaci in Israel--distribution, resistance to insecticides and implications for pest management. J Insect Sci 8
Hywel-Jones NL, Gillespie AT (1990) Effect of temperature on spore germination in Metarhizium anisopliae and Beauveria bassiana. Mycol Res 94:389–392
Jackson MA, Dunlap CA, Jaronski ST (2010) Ecological considerations in producing and formulating fungal entomopathogens for use in insect biocontrol. BioControl 55:129–145
Lacey LA, Fransen JJ, Carruthers R (1996) Global distribution of a naturally occurring fungi of Bemisia, their biologies and use as biological control agents. Bemisia 1995, Taxon Biol damage, Control Manag
Majeed MZ, Fiaz M, Ma CS, Afzal M (2017) Entomopathogenicity of three muscardine fungi, Beauveria bassiana, Isaria fumosorosea and Metarhizium anisopliae, against the Asian citrus psyllid, Diaphorina citri Kuwayama (Hemiptera: Psyllidae). Egypt J Biol Pest Control 27
Maniania NK (1991) Susceptibility of Chilo partellus Swinhoe (Lep., Pyralidae) eggs to entomopathogenic hyphomycetes. J Appl Entomol 112:53–58
Mora MAE, Castilho AMC, Fraga ME (2017) Classification and infection mechanism of entomopathogenic fungi. Arq Inst Biol (Sao Paulo) 84
Nazir T, Basit A, Hanan A et al (2019) In vitro pathogenicity of some entomopathogenic fungal strains against green peach aphid Myzus persicae (Homoptera: Aphididae). Agronomy 9:7
Oliveira MRV, Henneberry TJE, Anderson P (2001) History, current status, and collaborative research projects for Bemisia tabaci. Crop Prot 20:709–723
Sato H (1993) Effect of temperature on mycelial growth of three muscardine fungi. Trans 44th Meet Kanto Branch Jpn For Soc, 1993 103–104
Shah PA, Goettel MS (1999) Directory of microbial control products and services. Microb Control Div Soc Invertebr Pathol Gainesville, FL 31
Wang Z, Yao M, Wu Y (2009) Cross-resistance, inheritance and biochemical mechanisms of imidacloprid resistance in B-biotype Bemisia tabaci. Pest Manag Sci Former Pestic Sci 65:1189–1194
Wraight SP, Carruthers RI, Jaronski ST et al (2000) Evaluation of the entomopathogenic fungi Beauveria bassiana and Paecilomyces fumosoroseus for microbial control of the silverleaf whitefly, Bemisia argentifolii. Biol Control 17:203–217
Wraight SP, Inglis GD, Goettel MS (2007) Fungi. Field Manual of Techniques in Invertebrate Pathology.(ed. Lacey, LA, & Kaya, HK), Chapter IV–pp. 223-248
Yun H-G, Kim D-J, Gwak W-S et al (2017) Entomopathogenic fungi as dual control agents against both the pest Myzus persicae and phytopathogen Botrytis cinerea. Mycobiology 45:192–198
Zaki FN (1998) Efficiency of the entomopathogenic fungus, Beauveria bassiana (Bals), against Aphis crassivora Koch and Bemesia tabaci, Gennandius. J Appl Entomol 122:397–399
Zimmermann G (2007) Review on safety of the entomopathogenic fungi Beauveria bassiana and Beauveria brongniartii. Biocontrol Sci Technol 17:553–596
Acknowledgements
Authors thank Xiufen Yang and Lihua Guo of the Institute of Plant Protection, Chinese Academy of Agricultural Sciences for their valuable advice on the experimental protocols and for their technical assistance.
Funding
This research was funded by the National Key Research and Development Program of China (2017YFD0200900) and was partially supported by the Graduate School of Chinese Academy of Agricultural Sciences (GSCAAS) for providing the PhD fellowship for this study.
Author information
Authors and Affiliations
Contributions
AUK, TN, and DQ conceived and designed the experiments. MAG and TN collected the data. GHJ and TA analyzed the data. AUK and TA performed the experiment. AUK, TS, and YAA wrote the paper. DQ critically revised of the manuscript for intellectual content. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Keerio, A.U., Nazir, T., Abdulle, Y.A. et al. In vitro pathogenicity of the fungi Beauveria bassiana and Lecanicillium lecanii at different temperatures against the whitefly, Bemisia tabaci (Genn.) (Hemiptera: Aleyrodidae). Egypt J Biol Pest Control 30, 41 (2020). https://doi.org/10.1186/s41938-020-00247-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41938-020-00247-8