Abstract
Background
Whitefly, Bemisia tabaci (Genn.) (Hemiptera: Aleyrodidae), is one of the most harmful pests in greenhouses and in open fields.
Main body
The present study aimed to assess in vitro virulence of 3 entomopathogenic fungal strains (EPFs) of Lecanicillium lecanii 3 (V-3), 4 (V-4), and 5 (V-5) against the whitefly, Bemisia tabaci (Genn.) (Hemiptera: Aleyrodidae), by spray method. The 3 disparate bioassays were performed encompassed of conidial concentrations and fungal filtrate of the strains, V-3, V-4, and V-5, and also their binary combinations. In the filtrate bioassay, 2 ml of fungal filtrate of each strain was used. In the conidial bioassay, 3 disparate concentrations (1 × 105, 1 × 106, and 1 × 107 conidia ml−1) were used for each fungal strain, while in the binary combinations (1 ml filtrate + 1 ml conidia) of V-3 × V-3, V-4 × V-4, and V-5 × V-5 were used. Mortality rates against the whitefly were recorded on the 7th day. In the conidial bioassay, maximum mortality rates were found at V-3 strain (90.6%), V-4 strain (78.4%), and V-5 strain (83.6%) at the highest concentration (1 × 107 conidia ml−1) on the 7th day. In the filtrate bioassay, V-3 strain revealed a maximum mortality (93%), V-4 strain (85%), and then V-5 strain (87%) on the 7th day. Moreover, in the bioassay of binary combinations, the highest mortality rate of the whitefly was counted in V-3 × V-3 strain (84.6%), V-4 × V-4 strain (70.6%), and V-5 × V-5 strain (79.8%) on the 7th day.
Conclusion
All treatments had the potential to control B. tabaci significantly. In all bioassays, the V-3 strain was the extreme virulent, and the filtrate application of V-3 strain was the utmost impressive against B. tabaci.
Similar content being viewed by others
Background
Whitefly, Bemisia tabaci (Genn.) (Hemiptera: Aleyrodidae), is one of the most serious pests of crops that causes great losses (Oliveira et al. 2001). It causes direct destruction by sucking the plant sap, and also indirect destruction by encouraging the growth of black sooty mold on their honeydew secretions (Varma and Malathi 2003). It causes economic losses in many crops, including leafy vegetables, cotton, beans, and tomatoes (Fontes et al. 2012). The application of chemical pesticides leads to increase resistance and has a negative environmental impact that encourages the development of alternative pest management strategies, where microbial control plays a key role (Nazir et al. 2019b). Application of microbial control agents mainly entomopathogenic fungi isolates (EPFs) has been examined to control various orchard and field crop pests (Lacey and Shapiro-Ilan 2008). The use of EPFs in biological control is developing extremely due to food safety concerns, environmental awareness, and the failure of conventional chemicals (Shahid et al. 2012). EPF can play a significant role in biocontrol programs because of their pathogenic pathways, their wide range of hosts, and the control of economic insect pests such as the sucking insects (thrips, mites, aphids whiteflies, and mealybugs) (Khan et al. 2012). The use of biological control for pest control in greenhouses has been proven to be effective, and its application worldwide is growing steadily (Perdikis et al. 2008). The most promising EPF include Lecanicillium lecanii, Metarhizium anisopliae, and Beauveria bassiana (Saleh et al. 2016). L. lecanii is widely distributed and can cause a large-scale epizootic in tropical and subtropical regions as well as in humid and warm environments (del Prado et al. 2008). When the fungal conidia encounter the host, they attach to the epidermis through a hydrophobic mechanism and germinate under favorable conditions to form the germ tube (Inglis and Goettel 2001). During this process, the fungus produces several infection specific structures, including penetration pegs or appressoria, enabling the developing hyphae to enter the host integument (Ortiz-Urquiza and Keyhani 2013). L. lecanii has been documented to infect B. tabaci (Zhu and Kim 2011) causing a good mortality (Xie et al. 2019).
The present study was undertaken to evaluate the bioefficacy of L. lecanii strains against B. tabaci under laboratory conditions.
Main text
Materials and methods
Insect rearing
Adults of the whitefly, B. tabaci were collected from Langfang station, the research area of Institute of Plant Protection (IPP), Chinese Academy of Agricultural Sciences (CAAS), Beijing, China. The pest was reared on cotton plants under a controlled greenhouse at 25 ± 2 °C, 65% RH with a 14:10 (L:D) photoperiod.
Fungal culture and conidia suspension
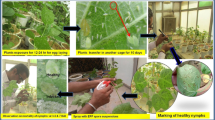
The L. lecanii strains, V3, V4, and V5, were obtained from the key laboratory of Biopesticides Engineering, Department of Bio-Pesticides and Biocontrol, Institute of Plant Protection (IPP), Chinese Academy of Agricultural Sciences (CAAS). The strains were obtained from infected B. tabaci. The strains were grown on Potatoes Dextrose Agar in Petri dishes for 20 days at 25 °C. Conidia were harvested after 21 days. The Petri dishes were flashed 20 ml with sterile water and filtered by using a sterile cheese cloth. Three different concentrations of conidia (1 × 105, 1 × 106, and 1 × 107) of spore suspension were determined using a hemocytometer. Earlier performing bioassays against B. tabaci, the viability test of conidia was accomplished, according to the procedures of (Hywel-Jones and Gillespie 1990).
Fungal filtrate
To get fungal filtrates, 4 ml of conidial suspension was prepared in 100 ml of Adamek liquid medium of all fungal strains. The culture was incubated 3 days in the dark for 150 rpm at 25 °C. Secondary culture was prepared and added to 3 ml of primary culture in to Adamek liquid medium and incubated 7 days in the dark for 150 rpm at 25 °C. The fungal filtrate was centrifuged 20 min, 11,000 rpm at 4 °C. The supernatant was filtered 0.45 μm pore size filter.
Pathogenicity test
The effects of the fungal conidia, filtrate, and binary combinations ((filtrate + conidia) on the cotton whitefly were tested under laboratory conditions using a manual sprayer. Three different conidia concentrations (1 × 105, 1 × 106, and 1 × 107) were tested. Filtrate bioassays were evaluated by applying 7 days fungal filtrate. Binary combinations (1 ml conidia + 1 ml filtrate) were estimated, while sterilized water was used as a control. For this purpose, 10 adults of whitefly were placed in a Petri dish for each treatment having fresh cotton leaves and incubated at 25 ± 2 °C 65% RH. Whitefly mortality rate was recorded on 3rd, 5th, 6th, and 7th day. Each treatment had 5 replications.
Statistical analysis
Virulence of the 3 stains of L. lecanii as conidia, filtrate, and binary combination were analyzed, using factorial analysis of variance (ANOVA) Statistix software (Version 8.1) (Tallahassee, FL). Comparison of the treatment means was performed using Fischer’s least significant difference (LSD) test at α = 0.05).
Results and discussion
Pathogenicity test of conidia
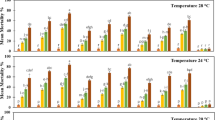
To evaluate the mortality of B. tabaci, 3 concentrations (1 × 105, 1 × 106, and 1 × 107) of strains of L. lecanii were tested under laboratory conditions. The factorial analysis of variance exhibited significant impact of disparate concentrations, disparate intervals of time, and their interaction on the mean whitefly mortality rates (F = 399.41, p ≤ 0.001; F = 297.14, p ≤ 0.001; and F = 16.42, p ≤ 0.001, respectively (Table 1)). Strain V-3 of L. lecanii caused a significant mortality rate of whitefly at all the concentrations than the control. Nonetheless, the maximum mortality (90.6%) was noted at 7th day at a concentration of 1 × 107 conidia ml−1. The minimum mortality (69.4%) was found at 7th day at a concentration of 1 × 105 conidia ml−1 (Fig. 1). Toxicity of L. lecanii strain V-5 against B. tabaci showed akin trend such as in the V-3. Maximum mortality rate (83.6%) of whitefly was witnessed after the 7th day at the concentration of 1 × 107 conidia ml−1 (Fig. 1). The minimum mortality (65.2%) was reported at a concentration of 1 × 105 conidia ml−1 (Fig. 1). The highest mortality rate (78.4%) of L. lecanii strain V-4 against B. tabaci was recorded at the highest concentration (1 × 107conidia ml−1), while the minimum mortality of whitefly (64%) was witnessed at the concentration of 1 × 105 conidia ml−1 at 7th day (Fig. 1). Mean whitefly mortality in the control Petri dishes was 16.2%.
Mean mortality (%) of Bemisia tabaci noted at different time intervals (3rd, 5th, 6th, and 7th) for conidial bioassays executed with different strains of Lecanicillium lecanii (V-3, V-4, and V-5) at three conidial concentrations (i.e., 1 × 105, 1 × 106, and 1 × 107 conidia ml−1) of each fungal strain and one control. The columns show mean percent mortality ± SE (n = 5). Treatment columns bearing different alphabets are significantly different from other treatments (LSD test at p = 0.05). See Table 1
Filtrate bioassay
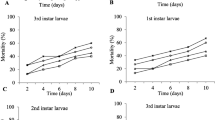
Outcomes of the filtrate bioassay showed that overall average mortality of the whitefly through the tested strains of fungus was more than the treatments of conidia. Filtrates of fungus, observation , and their interactions on B. tabaci mortality had a significant impact (Table 2). The maximum mortality (93%) was noted on the 7th day of treatment of (V-3) and the minimum mortality (85%) was noted on the 7th day through the application of filtrate of V-4. Filtrate of fungal strain V-5 revealed an average whitefly mortality rate of 87% (Fig. 2).
Mean mortality (%) of Bemisia tabaci noted at different time intervals (3rd, 5th, 6th, and 7th) for filtrate bioassays executed with different strains of Lecanicillium lecanii (V-3, V-4, and V-5). The columns show mean percent mortality ± SE (n = 5). Treatment columns bearing different alphabets are significantly different from other treatments (least significant difference (LSD test at p = 0.05). See Table 2
Binary combination (conidia + filtrate) virulence
The pathogenicity bioassays executed by the binary combinations (conidia + filtrate) of three strains of fungus had a significant impact of all the treatments, observation time, and their interactions on whitefly mortality (Table 3). Significant and maximum whitefly mortality (84.6%) was witnessed at V-3 × V-3, while the minimum mortality rate of whitefly (70.6%) was noted at the combination of V-4 × V-4 on the 7th day of treatment. Binary combination of V-5 × V-5 affected (79.8%) whitefly mortality on the 7th day of treatment (Fig. 3).
Mean mortality (%) of Bemisia tabaci noted at different time intervals (3rd, 5th, 6th, and 7th) for bioassays executed with the binary combinations of different strains of Lecanicillium lecanii. Treatments included the combination of V-3 × V-3, V-4 × V-4, and V-5 × V-5, and control. The columns shows mean percent mortality ± SE (n = 5). Treatment columns bearing different alphabets are significantly different from other treatments (LSD test at p = 0.05). See Table 3
Generally, the study outcomes are in line with the earlier studies describing the efficiency of disparate isolates of L. lecanii and Beauveria bassiana against the sucking insect pests (Hesketh et al. 2008).
In all the bioassays, the mortality rate of B. tabaci was reliant on concentration and time, and it increased through increasing the conidia concentration and time interval after the application. The mortality of 90.6, 78.4, and 83.6% was reported after 7 days of conidial application of V-3, V-4, and V-5 at the highest concentration 1 × 107 conidia ml−1 whereas 93, 85, and 87% mortality rate was recorded after 7 days of filtrate application of V-3, V-4, and V-5 strains. One more experiment was executed and stated 84.6, 70.6, and 79.8% mortality of B. tabaci after 7 days by binary combination (conidia + filtrate) of V-3 × V-3, V-4 × V-4, and V-5 × V-5 fungal strains. Obtained results are in line with (Halimona and Jankevica 2011) who verified several concentrations of five EPFs against A. fabae and M. fuscoviride and found that the highest concentration 1 × 108 ml−1 of conidia exhibited the maximum mortality after 7 days. Also, the results are in accordance with (Hanan et al. 2020b) who demonstrated that in all the bioassays, L. lecanii V-3 strain was the utmost pernicious and application of its filtrate was found to be the utmost efficient against green peach aphid Myzus persicae (Sulz.) (Nazir et al. 2019a).
Mortality percentage of B. tabaci affected with the conidial concentration, exposure time and temperature (Keerio et al. 2020). Similarly, Wright et al. (2005) also confirmed that the aphids mortality increased through an increase of the concentration of conidia and time interval. The V. lecanii revealed maximum mortality in initial stages and minimum mortality in the older instars of B. tabaci (Zaki 1998). EPF demonstrated a worthy control of whitefly (Abdel-Raheem et al. 2016). The application of filtrate was more efficient than the application of conidia chiefly in the insects having a short life cycle to control the insect pests. There could be a possible reason for this that the conidia attachment to the cuticle of insects is less effective than the enormous penetration of the filtrates (Hanan et al. 2020a).
Regarding combined applications, acquired results were similar to Yun et al. (2017) who revealed the dual action of EPF, M. anisopliae, B. bassiana, and Botrytis cinerea against M. persicae, and observed that the practice of filtrate by blastospores presented the extreme mortality of M. persicae. The binary combination (conidia + filtrate) provided the lowest mortality rate as compared to the application of filtrate. Thus, it is clear that the application of fungal filtrate had the utmost virulence efficiency.
Conclusions
The in vitro study revealed the competence of 3 strains of L. lecanii against B. tabaci. V-4 exhibited the lowest mortality rate than the other both strains (V-3 and V-5), either alone or in the binary combinations (conidia + filtrate) of identical strain. The filtrate application was extremely suitable material to control the whitefly. Binary combination of conidia + filtrate had some of the invincible effect and cannot be used efficiently to control whitefly. However, the most characterization of L. lecanii strains (V-3, V-4 and V-5) and their prospective functional insecticidal elicitors is required to better clarify their approach of action.
Availability of data and materials
All data and materials are mentioned in the manuscript.
Abbreviations
- PDA:
-
Potato dextrose agar
- L:D:
-
Light:dark
References
Abdel-Raheem MA, Reyad NF, Abdel-Rahman IE, Al-Shuraym L (2016) Evaluation of some isolates of entomopathogenic fungi on some insect pests infesting potato crop in Egypt. Int J Chem Tech Res 9:479–485
del Prado EN, Iannacone J, Gómez H (2008) Effect of two entomopathogenic fungi in controlling Aleurodicus cocois (Curtis, 1846)(Hemiptera: Aleyrodidae). Chil J Agric Res 68:21–30
Fontes F von HM, Colombo CA, Lourenção AL (2012) Structure of genetic diversity of Bemisia tabaci (Genn.)(Hemiptera: Aleyrodidae) populations in Brazilian crops and locations. Sci Agric 69:47–53.
Halimona J, Jankevica L (2011) The influence of Entomophthorales isolates on aphids Aphis fabae and Metopeurum fuscoviride. Latv Entomol 50:55–60
Hanan A, Basit A, Nazir T et al (2020a) Anti-insect activity of a partially purified protein derived from the entomopathogenic fungus Lecanicillium lecanii (Zimmermann) and its putative role in a tomato defense mechanism against green peach aphid. J Invertebr Pathol 170:107282
Hanan A, Nazir T, Basit A et al (2020b) Potential of Lecanicillium lecanii (Zimm.) as a microbial control agent for green peach aphid, Myzus persicae (Sulzer) (Hemiptera: Aphididae). Pak J Zool 52:131
Hesketh H, Alderson PG, Pye BJ, Pell JK (2008) The development and multiple uses of a standardised bioassay method to select hypocrealean fungi for biological control of aphids. Biol Control 46:242–255
Hywel-Jones NL, Gillespie AT (1990) Effect of temperature on spore germination in Metarhizium anisopliae and Beauveria bassiana. Mycol Res 94:389–392
Inglis GD, Goettel MS (2001) Use of Hyphomycetous fungi for managing insect pests. In: Butt TM, Jackson CY, Magan N (eds) Fungi as biocontrol agents: progress, problems and potential
Keerio AU, Nazir T, Abdulle YA et al (2020) In vitro pathogenicity of the fungi Beauveria bassiana and Lecanicillium lecanii at different temperatures against the whitefly, Bemisia tabaci (Genn.)(Hemiptera: Aleyrodidae). Egypt J Biol Pest Control 30:1–9
Khan S, Guo L, Maimaiti Y et al (2012) Entomopathogenic fungi as microbial biocontrol agent. Mol Plant Breed 3:63–79
Lacey LA, Shapiro-Ilan DI (2008) Microbial control of insect pests in temperate orchard systems: potential for incorporation into IPM. Annu Rev Entomol 53:121–144
Nazir T, Basit A, Hanan A et al (2019a) In vitro pathogenicity of some Entomopathogenic fungal strains against green peach aphid Myzus persicae (Homoptera: Aphididae). Agronomy 9:7
Nazir T, Khan S, Qiu D (2019b) Biological control of insect pest. In: Pests-insects, management, control. IntechOpen https://doi.org/10.5772/intechopen.81431
Oliveira MRV, Henneberry TJ e, Anderson P (2001) History, current status, and collaborative research projects for Bemisia tabaci. Crop Prot 20:709–723
Ortiz-Urquiza A, Keyhani N (2013) Action on the surface: entomopathogenic fungi versus the insect cuticle. Insects 4:357–374
Perdikis D, Kapaxidi E, Papadoulis G (2008) Biological control of insect and mite pests in greenhouse solanaceous crops. Eur J Plant Sci Biotechnol 2:125–144
Saleh MME, Abdel-Raheem MA, Ebadah IM, Huda HE (2016) Natural abundance of entomopathogenic fungi in fruit orchards and their virulence against Galleria mellonella larvae. Egypt J Biol Pest Control 26:203
Shahid AA, Rao QA, Bakhsh A, Husnain T (2012) Entomopathogenic fungi as biological controllers: new insights into their virulence and pathogenicity. Arch Biol Sci 64:21–42
Varma A, Malathi VG (2003) Emerging geminivirus problems: a serious threat to crop production. Ann Appl Biol 142:145–164
Wright MS, Raina AK, Lax AR (2005) A strain of the fungus Metarhizium anisopliae for controlling subterranean termites. J Econ Entomol 98:1451–1458
Xie T, Jiang L, Li J et al (2019) Effects of Lecanicillium lecanii strain JMC-01 on the physiology, biochemistry, and mortality of Bemisia tabaci Q-biotype nymphs. PeerJ 7:e7690
Yun H-G, Kim D-J, Gwak W-S et al (2017) Entomopathogenic fungi as dual control agents against both the pest Myzus persicae and phytopathogen Botrytis cinerea. Mycobiology 45:192–198
Zaki FN (1998) Efficiency of the entomopathogenic fungus, Beauveria bassiana (Bals), against Aphis crassivora Koch and Bemesia tabaci, Gennandius. J Appl Entomol 122:397–399
Zhu H, Kim JJ (2011) Susceptibility of the tobacco whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae) biotype Q to entomopathogenic fungi. Biocontrol Sci Tech 21:1471–1483
Acknowledgements
The authors thank the China Scholarship Council (CSC) and Graduate School of Chinese Academy of Agricultural Sciences (GSCAAS) for providing a Ph.D. scholarship.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Conceptualization: YAA, TN, and DQ. Data curation: YAA and TN. Formal analysis: TN, HA, and TA. Funding acquisition: DQ. Investigation: YAA, TN, and TDN. Methodology: YAA and AUK. Project administration: DQ. Resources: DQ. Software: YAA, TN, and SZ. Supervision: DQ. Validation: TN and AUK. Writing – original draft: YAA and AUK. Writing – review and editing: TN, HA, SZ, and DQ. The authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdulle, Y.A., Nazir, T., Keerio, A.U. et al. In vitro virulence of three Lecanicillium lecanii strains against the whitefly, Bemisia tabaci (Genn.) (Hemiptera: Aleyrodidae). Egypt J Biol Pest Control 30, 129 (2020). https://doi.org/10.1186/s41938-020-00328-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41938-020-00328-8