Abstract
Background
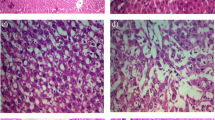
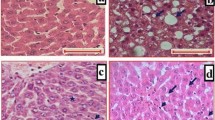
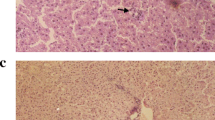
The management of nonalcoholic steatohepatitis (NASH) is still a crosstalk so the current study was designed to evaluate the effect of different luteolin doses on an experimental model of NASH and to elucidate novel anti-inflammatory pathways underlying its effect.
Methods
Adult male Wistar rats (200–220 g; n = 60) were used. Rats were fed a high carbohydrate/high fat diet (˜ 30% carbohydrate and 42% fat) daily for 12 weeks to induce NASH. Luteolin (10, 25, 50 or 100 mg/kg/day) was administered as a suspension (10% w/v in 0.9% NaCl) using an oral gavage. Histopathological changes (necrosis, inflammation and steatosis) were evaluated. Biomarkers for liver function, lipid peroxidation, extracellular matrix deposition and anti-oxidant activity were measured. Levels of IFN-γ, TNF-α and IL-1α and IL-18 were measured.
Results
Obtained results showed ability of luteolin to reduce activity of ALT and AST and to decrease levels of bilirubin, hyaluronic acid and malondialdehyde significantly (p < 0.05). Also, luteolin showed an anti-oxidant activity as indicated by the significant (p < 0.05) increase in reduced glutathione. Finally, a significant (p < 0.05) decrease in IFN-γ, TNF-α, IL-1α and IL-18 levels was observed most notably in groups that received high doses of luteolin (50 and 100 mg/kg).
Conclusions
Luteolin can protect against non-alcoholic steatohepatitis through targeting the pro-inflammatory IL-1 and Il-18 pathways in addition to an antioxidant effect.
Similar content being viewed by others
References
Yang ZR, Wang HF, Zuo TC, Guan LL, Dai N. Salidroside alleviates oxidative stress in the liver with non-alcoholic steatohepatitis in rats. BMC Pharmacol Toxicol 2016;17(16).
Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: mayo clinic experiences with a hitherto unnamed disease. Mayo Clin Proc 1980;55:434–8.
Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012;55:2005–23.
Wobser H, Dorn C, Weiss TS, Amann T, Bollheimer C, Büttner R, et al. Lipid accumulation in hepatocytes induces fibrogenic activation of hepatic stellate cells. Cell Res 2009;19:996–1005.
Arrese M, Cabrera D, Kalergis AM, Feldstein AE. Innate immunity and inflammation in NAFLD/NASH. Dig Dis Sci 2016;61:1294–303.
González-Gallego J, Sánchez-Campos S, Tuñón MJ. Anti-inflammatory properties of dietary flavonoids. Nutr Hosp 2007;22:287–93.
Trochon V, Mabilat-Pragnon C, Bertrand P, Legrand Y, Soria J, Soria C, et al. Hyaluronectin blocks the stimulatory effect of hyaluronan-derived fragments on endothelial cells during angiogenesis in vitro. FEBS Lett 1997;418:6–10.
Galati G, Teng S, Moridani MY, Chan TS, O’Brien PJ. Cancer chemoprevention and apoptosis mechanisms induced by dietary polyphenolics. Drug Metabol Drug Interact 2000;17:311–49.
Chen CY, Peng WH, Tsai KD, Hsu SL. Luteolin suppresses inflammation-associated gene expression by blocking NF-kappaB and AP-1 activation pathway in mouse alveolar macrophages. Life Sci 2007;81:1602–14.
Han DH, Denison MS, Tachibana H, Yamada K. Relationship between estrogen receptor-binding and estrogenic activities of environmental estrogens and suppression by flavonoids. Biosci Biotechnol Biochem 2002;66:1479–87.
Horinaka M, Yoshida T, Shiraishi T, Nakata S, Wakada M, Nakanishi R, et al. Luteolin induces apoptosis via death receptor 5 upregulation in human malignant tumor cells. Oncogene 2005;24:7180–9.
Zhang H, Tan X, Yang D, Lu J, Liu B, Baiyun R, et al. Dietary luteolin attenuates chronic liver injury induced by mercuric chloride via the Nrf2/NF-κB/P53 signalling pathway in rats. Oncotarget 2017;8:40982.
Domitrović R, Jakovac H, Milin Č, Radošević-Stašić B. Dose-and time-dependent effects of luteolin on carbon tetrachloride-induced hepatotoxicity in mice. Exp Toxicol Pathol 2009;61:581–9.
Li J, Li X, Xu W, Wang S, Hu Z, Zhang Q, et al. Antifibrotic effects of luteolin on hepatic stellate cells and liver fibrosis by targeting AKT/mTOR/p70S6K and TGFβ/Smad signalling pathways. Liver Int 2015;35:1222–33.
Chei S, Hwang JH, Lee YJ, Koh EJ, Choi J, Song JH, et al. Luteolin prevents palmitic acid-induced hepatic steatosis by regulating ER stress in HepG2. FASEB J 2017;31 (1 supplement) 972–23.
Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995;22:696–9.
Wahsh E, Abu-Elsaad N, El-Karef A, Ibrahim T. The vitamin D receptor agonist, calcipotriol, modulates fibrogenic pathways mitigating liver fibrosis in-vivo: an experimental study. Eur J Pharmacol 2016;789:362–9.
Patton HM, Patel K, Behling C, Bylund D, Blatt LM, Vallée M, et al. The impact of steatosis on disease progression and early and sustained treatment response in chronic hepatitis C patients. J Hepatol 2004;40:484–90.
Ibrahim SH, Hirsova P, Gores GJ. Non-alcoholic steatohepatitis pathogenesis: sublethal hepatocyte injury as a driver of liver inflammation. Gut 2018;67:963–72.
Panchal SK, Poudyal H, Iyer A, Nazer R, Alam A, Diwan V, et al. High-carbohydrate high-fat diet-induced metabolic syndrome and cardiovascular remodeling in rats. J Cardiovasc Pharmacol 2011;57:51–64.
Bahcecioglu IH, Yalniz M, Ataseven H, Ilhan N, Ozercan IH, Seckin D, et al. Levels of serum hyaluronic acid, TNF-alpha and IL-8 in patients with nonalcoholic steatohepatitis. Hepatogastroenterology 2005;52:1549–53.
Lydatakis H, Hager IP, Kostadelou E, Mpousmpoulas S, Pappas S, Diamantis I. Non-invasive markers to predict the liver fibrosis in non-alcoholic fatty liver disease. Liver Int 2006;26:864–71.
Tai M, Zhang J, Song S, Miao R, Liu S, Pang Q, et al. Protective effects of luteolin against acetaminophen-induced acute liver failure in mouse. Int Immunopharmacol 2015;27:164–70.
Alkhouri N, McCullough AJ. Noninvasive diagnosis of NASH and liver fibrosis within the spectrum of NAFLD. Gastroenterol Hepatol 2012;8:661–8.
Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology 1998;114:842–5.
Filozof C, Goldstein BJ, Williams RN, Sanyal A. Non-alcoholic steatohepatitis: limited available treatment options but promising drugs in development and recent progress towards a regulatory approval pathway. Drugs 2015;75:1373–92.
Kuo MY, Liao MF, Chen FL, YC Yang, ML Lin RH, et al. Luteolin attenuates the pulmonary inflammatory response involves abilities of antioxidation and inhibition of MAPK and NFκB pathways in mice with endotoxin-induced acute lung injury. Food Chem Toxicol 2011;49:2660–6.
Sahu BD, Kumar JM, Sistla R. Baicalein, a bioflavonoid, prevents cisplatin-induced acute kidney injury by up-regulating antioxidant defenses and down-regulating the MAPKs and NF-κB pathways. PLoS One 2015;10:e0134139.
Zhang Y, Tian XQ, Song XX, Ge JP, Xu YC. Luteolin protect against diabetic cardiomyopathy in rat model via regulating the AKT/GSK-3β signalling pathway. Biomed Res-India 2017;28:1359–63.
Lin Y, Shi R, Wang X, Shen HM. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr Cancer Drug Targets 2008;8:634–46.
Lebensztejn DM, Flisiak-Jackiewicz M, Białokoz-Kalinowska I, Bobrus-Chociej A, Kowalska I. Hepatokines and non-alcoholic fatty liver disease. Acta Biochim Pol 2016;63:459–67.
Wu R, Nakatsu G, Zhang X, Yu J. Pathophysiological mechanisms and therapeutic potentials of macrophages in non-alcoholic steatohepatitis. Expert Opin Ther Targets 2016;20:615–26.
Palomo J, Dietrich D, Martin P, Palmer G, Gabay C. The interleukin (IL)-1 cytokine family-balance between agonists and antagonists in inflammatory diseases. Cytokine 2015;76:25–37.
Banerjee M, Saxena M. Interleukin-1 (IL-1) family of cytokines: role in type 2 diabetes. Clin Chim Acta 2012;413:1163–70.
Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med 2007;13:851–6.
Sakurai T, He G, Matsuzawa A, Yu GY Maeda S, Hardiman G, et al. Hepatocyte necrosis induced by oxidative stress and IL-1 alpha release mediate carcinogen induced compensatory proliferation and liver tumorigenesis. Cancer Cell 2008;14:156–65.
Dube PH, Revell PA, Chaplin DD, Lorenz RG, Miller VL. A role for IL-1α in inducing pathologic inflammation during bacterial infection. Proc Natl Acad Sci 2001;98:10880–5.
Kamari Y, Shaish A, Vax E, Shemesh S, Kandel-Kfir M, Arbel Y, et al. Lack of interleukin-1 alpha or interleukin-1 beta inhibits transformation of steatosis to steatohepatitis and liver fibrosis in hypercholesterolemic mice. J Hepatol 2011;55:1086–94.
Hohenester S, Einer C, Wimmer R, Nagel J, Reiter FP, Schulz S, et al. Progression of non-alcoholic fatty liver disease towards steatohepatitis is differentially mediated by inflammasome-induced downstream pathways IL-1 and IL-18. J Hepatol 2016;64:S683–4.
Ibrahim SH, Hirsova P, Malhi H, Gores GJ. Animal models of nonalcoholic steatohepatitis: eat, delete, and inflame. Dig Dis Sci 2016;61:1325–36.
Membrez M, Ammon-Zufferey C, Philippe D, Aprikian O, Monnard I, Macé K, et al. Interleukin-18 protein level is upregulated in adipose tissue of obese mice. Obesity (Silver Spring) 2009;17:393–5.
Kaneda M, Kashiwamura SI, Ueda H, Sawada K, Sugihara A, Terada N, et al. Inflammatory liver steatosis caused by IL-12 and IL-18. J Interferon Cytokine Res 2003;23:155–62.
Sagawa H, Naiki-Ito A, Kato H, Naiki T, Yamashita Y, Suzuki S, et al. Connexin 32 and luteolin play protective roles in non-alcoholic steatohepatitis development and its related hepatocarcinogenesis in rats. Carcinogenesis 2015;36:1539–49.
Tan Q, Hu J, Yu X, Guan W, Lu H, Yu Y, et al. The role of IL-1 family members and Kupffer cells in liver regeneration. Biomed Res Int 2016;2016:6495793.
Bieghs V, Walenbergh S, Hendrikx T, et al. Trapping of oxidized LDL in lysosomes of Kupffer cells is a trigger for hepatic inflammation. Liver Int 2013;33:1056–61.
Ioannou GN. The role of cholesterol in the pathogenesis of NASH. Trends Endocrinol Metab 2016;27:84–95.
Abu-Elsaad N, El-Karef A. The falconoid luteolin mitigates the myocardial inflammatory response induced by high-carbohydrate/high-fat diet in wistar rats. Inflammation 2018;41:221–31.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abu-Elsaad, N., El-Karef, A. Protection against nonalcoholic steatohepatitis through targeting IL-18 and IL-1alpha by luteolin. Pharmacol. Rep 71, 688–694 (2019). https://doi.org/10.1016/j.pharep.2019.03.009
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/j.pharep.2019.03.009