Abstract
Background
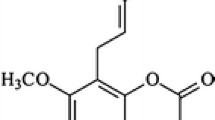
Intracerebral haemorrhage (ICH) as a devastating form of stroke has remained a public health threat due to lack of FDA-approved therapy. Oxidative stress originated from blood cell degradation products plays a crucial role in the ICH pathogenesis. In this study we evaluated oleuropein, a potent natural antioxidant from olive, in a well-established rat ICH model from overall symptoms to detailed molecular mechanism.
Methods
ICH model was established by collagenase injection to the brain of rats, which were randomly divided into groups with vehicle mock treatment, followed by treatment with different doses of oleuropein via daily intraperitoneal injection post-ICH for 3 days. The overall neurological deficit, brain edema level and blood-brain barrier (BBB) integrity were then measured in different treatment groups. To understand the protection mechanism of oleuropein in ICH, BBB structural components ZO-1 and occludin, oxidative stress and MAPK signalling pathways were also examined.
Results
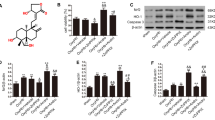
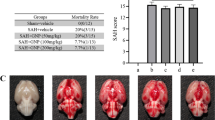
Oleuropein treatment showed overall alleviation of ICH-associated neurological deficit and brain edema in a dose dependent manner. Consistently, it could preserve the BBB structure and attenuate oxidative stress as well as ICH-induced MAPK activation in brain tissue.
Conclusion
Our study suggests oleuropein could be used as a promising therapeutic agent for ICH.
Similar content being viewed by others
References
Adeoye O, Broderick JP. Advances in the management of intracerebral hemorrhage. Nat Rev Neurol 2010;6(11):593–601.
Keep RF, Xiang J, Ennis SR, Andjelkovic A, Hua Y, Xi G, et al. Blood-brain barrier function in intracerebral hemorrhage. Acta Neurochir Suppl 2008;105:73–7.
Keep RF, Zhou N, Xiang J, Andjelkovic AV, Hua Y, Xi G. Vascular disruption and blood-brain barrier dysfunction in intracerebral hemorrhage. Fluids Barriers CNS 2014;11:18.
de Oliveira Manoel AL, Goffi A, Zampieri FG, Duggal D, Turkel-Parrella D, Marotta TR, et al. The critical care management of spontaneous intracranial hemorrhage: a contemporary review. Crit Care 2016;20:272.
Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis 2010;37(1):13–25.
Cardoso FL, Brites D, Brito MA. Looking at the blood-brain barrier: molecular anatomy and possible investigation approaches. Brain Res Rev 2010;64(2):328–63.
Duan X, Wen Z, Shen H, Shen M, Chen G. Intracerebral hemorrhage oxidative stress antioxidant therapy. Oxid Med Cell Longev 2016;2016:1203285.
Wang G, Yang Q, Li G, Wang L, Hu W, Tang Q, et al. Time course of heme oxygenase-1 and oxidative stress after experimental intracerebral hemorrhage. Acta Neurochir (Wien) 2011;153(2):319–25.
Hall NC, Packard BA, Hall CL, de Courten-Myers G, Wagner KR. Protein oxidation and enzyme susceptibility in white and gray matter with in vitro oxidative stress: relevance to brain injury from intracerebral hemorrhage. Cell Mol Biol (Noisy-le-grand) 2000;46(3):673–83.
Nakamura T, Keep RF, Hua Y, Park JW, Itano T, Nagao S, et al. Intracerebral hemorrhage induces edema and oxidative stress and alters N-methyl-D-aspartate receptor subunits expression. Acta Neurochir Suppl 2005;95:421–4.
Gaitanaki C, Konstantina S, Chrysa S, Beis I. Oxidative stress stimulates multiple MAPK signalling pathways and phosphorylation of the small HSP27 in the perfused amphibian heart. J Exp Biol 2003;206(Pt 16):2759–69.
Akasaka E, Takekoshi S, Horikoshi Y, Toriumi K, Ikoma N, Mabuchi T, et al. Protein oxidative damage and heme oxygenase in sunlight-exposed human skin: roles of MAPK responses to oxidative stress. Tokai J Exp Clin Med 2010;35(4):152–64.
Fujimoto S, Katsuki H, Ohnishi M, Takagi M, Kume T, Akaike A. Thrombin induces striatal neurotoxicity depending on mitogen-activated protein kinase pathways in vivo. Neuroscience 2007;144(2):694–701.
Wan S, Zhan R, Zheng S, Hua Y, Xi G. Activation of c-Jun-N-terminal kinase in a rat model of intracerebral hemorrhage: the role of iron. Neurosci Res 2009;63(2):100–5.
Puel C, Mathey J, Agalias A, Kati-Coulibaly S, Mardon J, Obled C, et al. Dose-response study of effect of oleuropein: an olive oil polyphenol, in an ovariectomy/inflammation experimental model of bone loss in the rat. Clin Nutr 2006;25(5):859–68.
Esmailidehaj M, Bajoovand S, Rezvani ME, Sherifidehaj M, Hafezimoghadam Z, Hafizibarjin Z. Effect of oleuropein on myocardial dysfunction and oxidative stress induced by ischemic-reperfusion injury in isolated rat heart. J Ayurveda Integr Med 2016;7(4):224–30.
Shi C, Chen X, Liu Z, Meng R, Zhao X, Liu Z, et al. Oleuropein protects L-02 cells against H2O2-induced oxidative stress by increasing SOD1, GPx1 and CAT expression. Biomed Pharmacother 2017;85:740–8.
Sun W, Wang X, Hou C, Yang L, Li H, Guo J, et al. Oleuropein improves mitochondrial function to attenuate oxidative stress by activating the Nrf2 pathway in the hypothalamic paraventricular nucleus of spontaneously hypertensive rats. Neuropharmacology 2017;113(Pt A):556–66.
Yu H, Liu P, Tang H, Jing J, Lv X, Chen L, et al. Oleuropein, a natural extract from plants, offers neuroprotection in focal cerebral ischemia/reperfusion injury in mice. Eur J Pharmacol 2016;775:113–9.
Casamenti F, Grossi C, Rigacci S, Pantano D, Luccarini I, Stefani M. A possible drug against degenerative conditions. In vivo evidence of its effectiveness against alzheimer’s disease. J Alzheimers Dis 2015;45(3):679–88.
Luccarini I, Ed Dami T, Grossi C, Rigacci S, Stefani M, Casamenti F. Oleuropein aglycone counteracts Abeta42 toxicity in the rat brain. Neurosci Lett 2014;558:67–72.
Shen LH, Li Y, Chen J, Cui Y, Zhang C, Kapke A, et al. One-year follow-up after bone marrow stromal cell treatment in middle-aged female rats with stroke. Stroke 2007;38(7):2150–6.
Kitaoka T, Hua Y, Xi G, Hoff JT, Keep RF. Delayed argatroban treatment reduces edema in a rat model of intracerebral hemorrhage. Stroke 2002;33(12):3012–8.
Zhong Z, Wang B, Dai M, Sun Y, Sun Q, Yang G, et al. Carvacrol alleviates cerebral edema by modulating AQP4 expression after intracerebral hemorrhage in mice. Neurosci Lett 2013;555:24–9.
Zhang ZY, Sun BL, Yang MF, Li DW, Fang J, Zhang S. Carnosine attenuates early brain injury through its antioxidative and anti-apoptotic effects in a rat experimental subarachnoid hemorrhage model. Cell Mol Neurobiol 2015;35(2):147–57.
Chen M, Lai L, Li X, Zhang X, He X, Liu W, et al. Baicalein attenuates neurological deficits and preserves blood-brain barrier integrity in a rat model of intracerebral hemorrhage. Neurochem Res 2016;41(11):3095–102.
Ohnishi M, Katsuki H, Fukutomi C, Takahashi M, Motomura M, Fukunaga M, et al. HMGB1 inhibitor glycyrrhizin attenuates intracerebral hemorrhage-induced injury in rats. Neuropharmacology 2011;61(5–6):975–80.
Ogun M, Ozcan A, Karaman M, Merhan O, Ozen H, Kukurt A, et al. Oleuropein ameliorates arsenic induced oxidative stress in mice. J Trace Elem Med Biol 2016;36:1–6.
Wei N, Wei Y, Li B, Pang L. Baicalein promotes neuronal and behavioral recovery after intracerebral hemorrhage via suppressing apoptosis, oxidative stress and neuroinflammation. Neurochem Res 2017;42:1345–53.
Xie RX, Li DW, Liu XC, Yang MF, Fang J, Sun BL, et al. Carnosine attenuates brain oxidative stress and apoptosis after intracerebral hemorrhage in rats. Neurochem Res 2016;42:541–51.
Zhai W, Chen D, Shen H, Chen Z, Li H, Yu Z, et al. A1 adenosine receptor attenuates intracerebral hemorrhage-induced secondary brain injury in rats by activating the P38-MAPKAP2-Hsp27 pathway. Mol Brain 2016;9(1):66.
Qureshi AI. The importance of acute hypertensive response in ICH. Stroke 2013;44(6 Suppl 1):S67–9.
Manaenko A, Chen H, Zhang JH, Tang J. Comparison of different preclinical models of intracerebral hemorrhage. Acta Neurochir Suppl 2011;111:9–14.
Aronowski J, Zhao X. Molecular pathophysiology of cerebral hemorrhage: secondary brain injury. Stroke 2011;42(6):1781–6.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shi, J., Wu, G., Zou, X. et al. Oleuropein protects intracerebral hemorrhage-induced disruption of blood-brain barrier through alleviation of oxidative stress. Pharmacol. Rep 69, 1206–1212 (2017). https://doi.org/10.1016/j.pharep.2017.05.004
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/j.pharep.2017.05.004