Abstract
Background
Oxidative stress and neurocyte apoptosis are crucial pathophysiological process in early brain injury (EBI) after subarachnoid hemorrhage (SAH). Geniposide (GNP) has been reported to exert neuroprotective effects by reducing oxidative injury and neurocyte apoptosis. However, the effect of GNP has not been clarified in EBI after SAH. The study was performed to evaluate the neuroprotective effects and mechanisms of GNP in EBI after SAH.
Methods and results
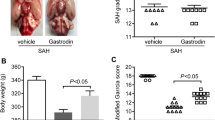
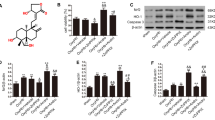
A total of 60 male Wistar rats were randomly divided into five groups. The prechiasmatic cistern SAH model was used in this study. SAH grade was evaluated using a grading system. Neurological function was evaluated using the Garcia scores. Brain edema was measured by the wet-dry method. Blood–brain barrier (BBB) permeability was measured by the extravasation of Evans Blue (EB). The neurocyte apoptosis was observed using TUNEL assay. The levels of malondialdehyde (MDA) and superoxide dismutase (SOD), as well as the expressions of nuclear factor erythroid 2-related factor 2 (Nrf2), hemeoxygenase-1 (HO-1), glutathione S-transferase (GST) and quinone oxidoreductase-1 (NQO-1) were performed. The results showed that GNP reduced brain edema, attenuated BBB permeability, inhibited neurocyte apoptosis and improved neurological function. Moreover, GNP also decreased the levels of ROS and MDA, elevated Nrf2 expression in the temporal cortex and up-regulated the expression of NQO-1, HO-1 and GST after SAH.
Conclusions
GNP could ameliorate oxidative stress and neurocyte apoptosis to exert neuroprotective effects by Nrf2 pathway.







Similar content being viewed by others
References
Lawton MT, Vates GE (2017) Subarachnoid hemorrhage. N Engl J Med 377:257–266. https://doi.org/10.1056/NEJMcp1605827
Chen S, Xu P, Fang Y, Lenahan C (2020) The updated role of the blood brain barrier in subarachnoid hemorrhage: from basic and clinical studies. Curr Neuropharmacol 18:1266–1278. https://doi.org/10.2174/1570159x18666200914161231
Yang Y, Chen S, Zhang JM (2017) The updated role of oxidative stress in subarachnoid hemorrhage. Curr Drug Deliv 14:832–842. https://doi.org/10.2174/1567201813666161025115531
Hasegawa Y, Suzuki H, Sozen T, Altay O, Zhang JH (2011) Apoptotic mechanisms for neuronal cells in early brain injury after subarachnoid hemorrhage. Acta Neurochir Suppl 110:43–48. https://doi.org/10.1007/978-3-7091-0353-1_8
Chen S, Feng H, Sherchan P, Klebe D, Zhao G, Sun X, Zhang J, Tang J, Zhang JH (2014) Controversies and evolving new mechanisms in subarachnoid hemorrhage. Prog Neurobiol 115:64–91. https://doi.org/10.1016/j.pneurobio.2013.09.002
Bellezza I, Giambanco I, Minelli A, Donato R (2018) Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta Mol Cell Res 1865:721–733. https://doi.org/10.1016/j.bbamcr.2018.02.010
Ahmed SM, Luo L, Namani A, Wang XJ, Tang X (2017) Nrf2 signaling pathway: pivotal roles in inflammation. Biochim Biophys Acta Mol Basis Dis 1863:585–597. https://doi.org/10.1016/j.bbadis.2016.11.005
Zhou YX, Zhang RQ, Rahman K, Cao ZX, Zhang H, Peng C (2019) Diverse pharmacological activities and potential medicinal benefits of geniposide. Evid Based Complement Alternat Med 2019:4925682. https://doi.org/10.1155/2019/4925682
Ma J, Ding Y (2018) Geniposide suppresses growth, migration and invasion of MKN45 cells by down-regulation of lncRNA HULC. Exp Mol Pathol 105:252–259. https://doi.org/10.1016/j.yexmp.2018.08.011
Lv C, Liu X, Liu H, Chen T, Zhang W (2014) Geniposide attenuates mitochondrial dysfunction and memory deficits in APP/PS1 transgenic mice. Curr Alzheimer Res 11:580–587. https://doi.org/10.2174/1567205011666140618095925
Lu W, Zhao Y, Kong Y, Zhang W, Ma W, Li W, Wang K (2018) Geniposide prevents H(2) O(2) -induced oxidative damage in melanocytes by activating the PI3K-Akt signalling pathway. Clin Exp Dermatol 43:667–674. https://doi.org/10.1111/ced.13409
Cheng S, Zhou F, Xu Y, Liu X, Zhang Y, Gu M, Su Z, Zhao D, Zhang L, Jia Y (2019) Geniposide regulates the miR-101/MKP-1/p38 pathway and alleviates atherosclerosis inflammatory injury in ApoE(-/-) mice. Immunobiology 224:296–306. https://doi.org/10.1016/j.imbio.2018.12.005
Liu F, Wang Y, Yao W, Xue Y, Zhou J, Liu Z (2019) Geniposide attenuates neonatal mouse brain injury after hypoxic-ischemia involving the activation of PI3K/Akt signaling pathway. J Chem Neuroanat 102:101687. https://doi.org/10.1016/j.jchemneu.2019.101687
Cheng F, Ma C, Sun L, Zhang X, Zhai C, Li C, Zhang S, Ren B, Liu S, Liu S, Yin X, Wang X, Wang Q (2018) Synergistic neuroprotective effects of Geniposide and ursodeoxycholic acid in hypoxia-reoxygenation injury in SH-SY5Y cells. Exp Ther Med 15:320–326. https://doi.org/10.3892/etm.2017.5395
Li S, Xiao X, Ni X, Ye Z, Zhao J, Hang C (2017) Tetramethylpyrazine Protects against Early Brain Injury after Experimental Subarachnoid Hemorrhage by Affecting Mitochondrial-Dependent Caspase-3 Apoptotic Pathway. Evid Based Complement Alternat Med 2017:3514914. https://doi.org/10.1155/2017/3514914
Chen JH, Wu T, Xia WY, Shi ZH, Zhang CL, Chen L, Chen QX, Wang YH (2020) An early neuroprotective effect of atorvastatin against subarachnoid hemorrhage. Neural Regen Res 15:1947–1954. https://doi.org/10.4103/1673-5374.280326
Sugawara T, Ayer R, Jadhav V, Zhang JH (2008) A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J Neurosci Methods 167:327–334. https://doi.org/10.1016/j.jneumeth.2007.08.004
Mo J, Enkhjargal B, Travis ZD, Zhou K, Wu P, Zhang G, Zhu Q, Zhang T, Peng J, Xu W, Ocak U, Chen Y, Tang J, Zhang J, Zhang JH (2019) AVE 0991 attenuates oxidative stress and neuronal apoptosis via Mas/PKA/CREB/UCP-2 pathway after subarachnoid hemorrhage in rats. Redox Biol 20:75–86. https://doi.org/10.1016/j.redox.2018.09.022
Zhuang K, Zuo YC, Sherchan P, Wang JK, Yan XX, Liu F (2019) Hydrogen inhalation attenuates oxidative stress related endothelial cells injury after subarachnoid hemorrhage in rats. Front Neurosci 13:1441. https://doi.org/10.3389/fnins.2019.01441
Zhang T, Wu P, Budbazar E, Zhu Q, Sun C, Mo J, Peng J, Gospodarev V, Tang J, Shi H, Zhang JH (2019) Mitophagy reduces oxidative stress via Keap1 (Kelch-Like epichlorohydrin-associated protein 1)/Nrf2 (nuclear factor-E2-related factor 2)/PHB2 (prohibitin 2) pathway after subarachnoid hemorrhage in rats. Stroke 50:978–988. https://doi.org/10.1161/strokeaha.118.021590
Pang J, Chen Y, Kuai L, Yang P, Peng J, Wu Y, Chen Y, Vitek MP, Chen L, Sun X, Jiang Y (2017) Inhibition of blood-brain barrier disruption by an apolipoprotein E-mimetic peptide ameliorates early brain injury in experimental subarachnoid hemorrhage. Transl Stroke Res 8:257–272. https://doi.org/10.1007/s12975-016-0507-1
Zhang X, Wu Q, Lu Y, Wan J, Dai H, Zhou X, Lv S, Chen X, Zhang X, Hang C, Wang J (2018) Cerebroprotection by salvianolic acid B after experimental subarachnoid hemorrhage occurs via Nrf2- and SIRT1-dependent pathways. Free Radic Biol Med 124:504–516. https://doi.org/10.1016/j.freeradbiomed.2018.06.035
Wu Q, Zhang XS, Wang HD, Zhang X, Yu Q, Li W, Zhou ML, Wang XL (2014) Astaxanthin activates nuclear factor erythroid-related factor 2 and the antioxidant responsive element (Nrf2-ARE) pathway in the brain after subarachnoid hemorrhage in rats and attenuates early brain injury. Mar Drugs 12:6125–6141. https://doi.org/10.3390/md12126125
Funding
This study was supported by Project of Administration of Traditional Chinese Medicine of Guangdong Province of China (No.20211157).
Author information
Authors and Affiliations
Contributions
S-X L designed the experiments. S-X S, Y-B L, X-C C, S-B W and J-J Z performed the material preparation and experiments. X-L X and A-L L analyzed the data and wrote the manuscript. S-X L and JC revised the manuscript. All authors approved the publication the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no potential conflicts of interest.
Ethical approval
Animal studies were conducted in accordance with the requirements of the directory of the Ethical Treatment of Experimental Animals of China. This study was approved by the Ethics Committee of the Guangdong Provincial Hospital of Chinese Medicine.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xiao, X., Sun, S., Li, Y. et al. Geniposide attenuates early brain injury by inhibiting oxidative stress and neurocyte apoptosis after subarachnoid hemorrhage in rats. Mol Biol Rep 49, 6303–6311 (2022). https://doi.org/10.1007/s11033-022-07438-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07438-6