Abstract
Abscission refers to the natural separation of plant structures from their parent plants, regulated by external environmental signals or internal factors such as stress and aging. It is an advantageous process as it enables plants to shed unwanted organs, thereby regulating nutrient allocation and ensuring the dispersal of fruits and seeds from the parent. However, in agriculture and horticulture, abscission can severely reduce crop quality and yield. In this review, we summarize the recent advances in plant abscission from the perspectives of developmental and molecular biology, emphasizing the diverse regulatory networks across different plant lineages, from model plants to crops. The sophisticated process of plant abscission involves several overlapping steps, including the differentiation of the abscission zone, activation of abscission, tissue detachment, and formation of a protective layer. Finally, we discuss the potential applications of physiological modifications and genetic manipulations of plant abscission in sustainable agriculture in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The word “abscission” refers to “removal or cutting away”, derived from the Latin “abscissionem”. In botany, it refers to the separation of plant structures, such as leaves, branches, flowers, or fruits, away from the parent plant owing to environmental changes (Estornell et al. 2013). These changes can be induced by a series of developmental (i.e., aging or maturation) or external environmental signals, including abiotic (drought, dark, hypoxia, extreme temperature, and nutrition limitation) and biotic stresses (mainly diseases or pests) (Reichardt et al. 2020; Li et al. 2021a; Goto et al. 2022; Meng et al. 2023; Ruiz et al. 2001; Patharkar et al. 2017).
From the perspectives of ecology and evolution, abscission is a beneficial process as it helps the parent plant discard unwanted parts such as wilted flowers or leaves, and hence regulates nutrient allocation. For example, leaf abscission can be triggered by drought, which may enable the plant to prepare well for subsequent occurrences of drought by reducing the leaf area for transpiration. Mobile nutrients, including those belonging to the three main nutrients classes (nitrogen, phosphorus, and potassium), are drawn out of the unhealthy old leaves before abscission to facilitate the continued growth of healthy young tissues (Patharkar and Walker 2016). In addition, leaf abscission can be triggered by pathogens in Arabidopsis thaliana, enabling the plants to shed infected leaves and eliminate the spread of the disease to healthy tissues (Patharkar et al. 2017). In forest and savanna ecosystems, abscised leaf litter plays key roles in nutrient and carbon cycling and forms a protective layer on the soil surface, thereby regulating the soil microclimate (Villalobos-Vega et al. 2011; Zhou et al. 2019). Abscission is also a key strategy for plant reproductive success, as it ensures the separation of fruits, which further crack to disperse the seeds. The seed abscission process largely relies on the wind, an important dispersal vector (Ferrándiz 2002; Schippers and Jongejans 2005).
However, in agriculture and horticulture, abnormal abscission is closely associated with severe reduction in crop quality and yield. In wild species of domesticated crops, seed shattering is an essential characteristic to ensure the survival of the next generation, whereas it causes major yield loss in crops harvested by humans. Therefore, our ancient farmers domesticated these wild species by collecting seeds from plants with favorable traits, including the loss of shattering, and produced non-shattering cultivated crops (Alam and Purugganan 2024). In addition, many fruit trees such as apple, pear, litchi, and citrus, suffer from flower and fruit abscission, which functions as a double-edged sword: excessive abscission leads to yield loss, while rational control of shedding may increase yield and improve fruit quality (Kon et al. 2023; Webster 2002; Zhao and Li 2020; Dutta et al. 2023).
Here, we review the recent advances in plant abscission from the perspectives of developmental and molecular biology, with an emphasis on the diverse regulatory networks in different plant lineages, from model plants to crops (Fig. 1; Table 1). The abscission process consists of several overlapping steps, including differentiation of the abscission zone, activation of abscission, tissue detachment, and formation of a protective layer (Fig. 1a). In the final part, we discuss the potential applications of physiological modifications and genetic manipulations of plant abscission for crop breeding. This review aims to enrich our understanding of the molecular regulatory networks involved in plant abscission and provide guidance for sustainable agriculture in the future.
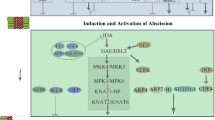
The process and molecular control of plant abscission. a Three overlapped steps during floral organ abscission in Arabidopsis. Abscission zone: AZ; se: sepal; pe: petal; st: stamen; green circles: other cells; red circles: differentiated cells in AZ; yellow circles: activated cells in AZ; blue circles: transdifferentiated cells on the surface of receptacle. (b-d) Molecular control of plant abscission in Arabidopsis thaliana (b), Solanum lycopersicum (c) and Oryza sativa (d). Dashed green lines: hypothetical pathways
1.1 Where to drop: the abscission zone
The location where abscission occurs, known as the abscission zone (AZ), is determined in the early developmental stages. AZs are present in various plant structures, including petioles, pedicels, and floral organs. Cell morphology in the AZ typically exhibits characteristics such as a smaller size compared to neighboring non-abscessed cells, denser protoplasm, increased cell density, and more complex plasmodesmata (Sexton and Roberts 1982). The number of cell layers in the AZ varies significantly across tissues and species. The floral organs of A. thaliana have 4-6 layers of cells in their AZ (McKim et al. 2008), tomato pedicels have 5-10 layers (Roberts et al. 1984), and the AZ at the leafstalks of Sambucus nigra consists of 50 layers of cells (Taylor and Whitelaw 2001). However, actual cell separation does not occur uniformly across the AZ but is typically limited to several distal cell layers, referred to as the abscission layer (Roberts et al. 1984). The abscission process has been described as a multistage process: (1) differentiation of the AZ, (2) activation of the abscission process in response to developmental and environmental signals, and (3) cell wall modification, followed by cell detachment and the formation of a rigid protective layer (Patterson 2001; Estornell et al. 2013).
1.2 Differentiation of the AZ
Many genes involved in the formation of AZ within floral organs, such as petals, sepals, and stamens, have been identified in Arabidopsis (Fig. 1a, b). BLADE-ON-PETIOLE 1/2 (BOP1/2) are NONEXPRESSOR OF PATHOGENESIS RELATED GENES 1 (NPR1)-like genes that redundantly regulate plants developmental patterning and facilitate the formation of AZ (Hepworth et al. 2005; Su et al. 2023). The bop1 bop2 double mutant fails to develop the anatomical structures of the AZ at the floral organ boundaries, leading to defects in organ abscission (McKim et al. 2008). In tobacco, a homolog of BOP, NtBOP2, regulates corolla abscission by inhibiting the longitudinal elongation of cells in the corolla AZ (Wu et al. 2012). Similarly, in tomatoes, CRISPR mutants of three BOP genes result in the failure of petal abscission (Xu et al. 2016). In legume species, Medicago truncatula, Pisum sativum and Lotus japonicus, BOP orthologs are necessary for the abscission of vegetative and reproductive structures (Couzigou et al. 2016). These findings support the important, conserved function of BOP in promoting AZ differentiation in eudicots.
Arabidopsis BOP1 and BOP2 form homodimers or heterodimers that enable them to activate the transcription of ASYMMETRIC LEAVES 2 (AS2) during leaf development (Jun et al. 2010). AS2 encodes an LBD transcription factor that acts in conjunction with the MYB transcription factor AS1. AS1 is also involved in the proper placement of the floral organ AZs and, together with AS2, forms a transcriptional complex that specifically binds to the CWGTTD motifs in the promoters of KNOTTED1-LIKE HOMEODOMAIN (KNOX) genes, such as KNOTTED-LIKE FROM ARABIDOPSIS THALIANA 1/2/6 (KNAT1/2/6, KNAT1 is also known as BREVIPEDICELLUS (BP)), resulting in the repression of their expression (Guo et al. 2008). KNAT1 inhibits floral organ abscission by limiting AZ cell size and number (Shi et al. 2011). In tomatoes, the KNOX gene KD1 is involved in the regulation of tomato flower pedicel abscission via the modulation of auxin concentration and response in the AZ (Ma et al. 2015). In Litchi chinensis, a tropical fruit originating from south China, LcKNAT1 is expressed in the fruitlet AZ, and ectopic expression of LcKNAT1 in tomatoes leads to delayed pedicel abscission (Zhao et al. 2020). These results reveal a shared role of KNOX proteins as negative abscission regulators. In addition, BOP1/2 can form complexes with the transcription factors TGACG-BINDING FACTOR 1/4 (TGA1/4), leading to the direct activation of the BEL1-LIKE (BELL) gene, ARABIDOPSIS THALIANA HOMEOBOX GENE1 (ATH1) (Khan et al. 2015). Both BELL and KNOX proteins belong to the three-amino-acid loop extension (TALE) protein family and share similar structures and functions in diverse developmental processes. ATH1 positively regulates stamen abscission and, together with its partners KNAT2/6, contribute to the differentiation of floral organ AZs (Crick et al. 2022). Additionally, TALE homeodomain transcription factors (ATH1, KNAT2/6) and BOP1/2 work together during lignin deposition to promote the expression of hydrolytic enzymes involved in cell separation (Crick et al. 2022). BOP1/2 contribute to cell separation via activation of ATH1 and KNAT2/6 or independently through the promotion of genes involved in cell separation (Crick et al. 2022).
Abscission occurs not only within floral organs but also in whole flowers, fruits, and branches. The tomato has been used as a model to study the mechanisms of AZ formation within the pedicel, where aborted flowers or ripe fruits are shed (Fig. 1c). Formation of the pedicel AZ in tomato requires the presence of at least three MADS-box transcription factors: JOINTLESS (J), JOINTLESS-2 (J-2, also known as SlMBP21), and MACROCALYX (MC) (Nakano et al. 2012; Roldan et al. 2017). Loss-of-function mutations in any of these genes result in the failure of pedicel AZ development. As MADS-box transcription factors assemble into core tetrameric protein complexes in the floral quartet model, J, J-2, and MC may also form tetramers with other MADS-box proteins, thereby serving as transcriptional activators that promote the development of the pedicel AZ (Liu et al. 2014). J, J-2, and MC activate the expression of pre-abscission-related genes, such as BLIND (Bl), GOBLET (GOB), Lateral suppressor (Ls), and a WUSCHEL homologue in tomato (LeWUS) (Nakano et al. 2012; Roldan et al. 2017; Nakano et al. 2013). The transcription of J is activated by BEL1-LIKE HOMEODOMAIN 4 (SlBL4) in vitro, supporting SlBL4’s role in fruit pedicel organogenesis and abscission (Yan et al.2021). However, the orthologs of these three MADS proteins in Arabidopsis are not related to pedicel abscission, and it remains unclear whether the functions of MADS transcription factors in the regulation of pedicel AZ development are conserved in other species or if they are specific to tomato and its relatives.
Preharvest fruit shattering occurs in many wild relatives of Poaceous crops, causing reduced yield and seed quality. Therefore, natural mutants with non-shattering trait were often selected during crop domestication (Yu et al. 2024). Although shattering positions vary among different Poaceous crops, they are often related to structures such as floral bracts and stem segments (Yu et al. 2024). In rice, the AZ consists of a layer of non-lignified cells surrounded by thick lignified cells (Wu et al. 2023b), and numerous shattering factors have been identified, including eight major factors: SUPERNUMERARY BRACT (SNB), QTL OF SEED SHATTERING IN CHROMOSOME 1 (qSH1), GRAIN SHATTERING QUANTITATIVE TRAIT LOCUS ON CHROMOSOME 4 (SH4), SH5, SHATTERING ABORTION1 (SHAT1), ORYZA SATIVA CTD PHOSPHATASE-LIKE 1 (OsCPL1), ORYZA BARTHII SEED SHATTERING 3 (ObSH3), and OgSH11 (Fig. 1d). These factors form a complicated network that regulates the expression of key lignin biosynthesis genes: GOLD HULL AND INTERNODE2 (GH2)/CINNAMYL-ALCOHOL DEHYDROGENASE 2 (CAD2) and 4-COUMARATE: COENZYME A LIGASE 3 (4CL3) (Wu et al. 2023a; Wu et al. 2023b). Notably, differential lignification that occurs in rice AZ formation may not be essential for shattering in many other Poaceae crops, such as sorghum and wheat, suggesting divergent genetic control of cereal shattering.
1.3 Activation of the abscission process
In A. thaliana, the IDA-HAE/HSL2 signaling pathway regulates the initiation of floral organ abscission (Fig. 1b). HEASA (HAE) and HEASA-LIKE2 (HSL2) encode two closely related leucine-rich repeating receptor-like kinases (LRR-RLKs) that redundantly regulate the process. INFLORESCENCE DEFICIENT IN ABSCISSION (IDA) and IDA-LIKE (IDL) are small peptides that positively regulate floral organ abscission process (Jinn et al. 2000; Stenvik et al. 2008). The ida mutants, despite possessing the ability to form AZ, fail to abscise floral organs, whereas ectopic expression of IDA results in earlier abscission (Butenko et al. 2003). IDA functions as a ligand to activate the heterodimerization and transphosphorylation of HAE/HSL2, together with SOMATIC EMBRYOGENESIS RECEPTOR KINASE (SERK) family members that function as co-receptors (Meng et al. 2016). The IDA-HAE/HSL2 signaling module has been identified in different species, including tomato, tobacco, soybean, citrus, rose, and litchi, indicating its conservation across eudicots (Lu et al. 2023; Ventimilla et al. 2021; Tucker and Yang 2012; Estornell et al. 2015; Singh et al. 2023; Ma et al. 2024; Wang et al. 2019a; Ying et al. 2016). Future studies should focus on the crosstalk between the IDA-HAE/HSL2 signaling module and plant abscission hormones (i.e., ethylene/ET) to fully understand the ET-dependent and -independent abscission pathways (Meir et al. 2019).
The activated HAE/HSL2-SERK complex subsequently induces the downstream mitogen-activated protein kinase (MAPK) signaling cascade through brassinosteroid signaling kinases (BSKs) and the MAPKKK, YODA (YDA, also known as MAPKKK4) (Galindo-Trigo et al. 2024a). The activated MAPK cascade further phosphorylates the downstream transcription factors and ultimately enhances hydrolase activity, thereby facilitating floral organ abscission (Cho et al. 2008). The MADS-box transcription factor AGAMOUS-LIKE15 (AGL15) is a direct target of MAPK. AGL15 acts as a negative regulator of HAE before phosphorylation but becomes a positive regulator when phosphorylated, thereby establishing a feedback loop for the regulation of floral organ abscission (Patharkar and Walker 2015). Another DNA binding with one finger (DOF) transcription factor, AtDOF4.7, is also a direct target of the MAPK cascade, which enables AtDOF4.7 to directly repress the expression of the abscission-related polygalacturonase (PG) gene, PGAZAT (Wang et al. 2016). In addition, ZINC FINGER PROTEIN2 (AtZFP2), a zinc-finger protein, is specifically expressed in the floral AZ, and its overexpression leads to delayed abscission. AtDOF4.7, which forms a transcriptional complex with AtZFP2, enhances the repression of PGAZAT (Wei et al. 2010). Moreover, HAE/HSL2 and other RLKs must be transported through the intracellular membrane system, an ADP-ribosylation factor, The GTPase-activating protein NEVERSHED (NEV), located in the trans-Golgi network (TGN), is required. Mutations in nev disrupt the TGN structure of the cells within the AZ, leading to organ abscission failure (Liljegren et al. 2009).
1.4 Cell detachment and protective layer formation
In response to an abscission signal, the cells in the AZ secrete enzymes, including cellulases (CELs), polygalacturonases (PGs), and xyloglucan endotransglucosylase/hydrolases (XTHs), that modify and hydrolyze the cell wall (Wu et al. 2024). These enzymes degrade cell walls between adjacent cell layers, ultimately resulting in cell detachment.
CEL is responsible for cellulose degradation. In Arabidopsis, AtCEL6 promotes silique dehiscence by facilitating cell separation in the abscission layer (He et al. 2018). Six cellulase genes (SlCEL1-6) have been identified in tomato, all of which are associated with ET-dependent floral abscission (Del Campillo and Bennett 1996). In litchi, the HD-Zip transcription factor LcHB2 acts as a positive regulator of fruit abscission by directly activating the cellulase genes LcCEL2 and LcCEL8 (Li et al. 2019).
PG catalyzes the degradation of pectin. Floral organ abscission in Arabidopsis depends on the presence of PGs, and mutations in PGAZAT, ARABIDOPSIS DEHISCENCE ZONE POLYGALACTURONASE1 (ADPG1) and QUARTET2 (QRT2) lead to delayed fruit dehiscence (Ogawa et al. 2009). Hence, both PGAZAT and QRT2 have been used as abscission markers (González-Carranza et al. 2007). Organ abscission is regulated by a combination of jasmonic acid (JA), ET, and abscission acid (ABA), which, in part, promotes the expression of QRT2 (Ogawa et al. 2009). Tomato PGs, such as TAPG1/2/4/5, are specifically expressed during the ET-induced abscission of leaves and flowers. Their expression is promoted by the APETALA2/ethylene responsive factor (AP2/ERF) transcription factor SlERF52, which acts downstream of J and MC (Kalaitzis et al. 1997; Nakano et al. 2014). Under drought stress, the small signaling peptide hormone, phytosulfokine (PSK) can induce an elevated expression of TAPG4, thereby promoting the abscission of tomato flowers and fruits (Reichardt et al. 2020).
XTH disrupts the xyloglucan chains and remodels the cellulose-xyloglucan complex structure. Accumulation of XTH in the AZ has been observed during abscission in various species, such as Arabidopsis, tomato, rose, litchi, cherry, and soybean, indicating the potential importance of XTH in organ abscission (Lashbrook et al. 2008; Tsuchiya et al. 2015; Singh et al. 2013; Ma et al. 2021a; Qiu et al. 2021; Tucker et al. 2007). Studies have also demonstrated the ET-responsiveness of the transcription of XTHs during abscission. In litchi, two ETHYLENE INSENSITIVE 3-LIKE (EIL) homologs, LcEIL2 and LcEIL3, which function as core transcription factors that activate various ET responses, directly activate the XTH genes LcXTH4/7/19, and mediate fruit abscission (Ma et al. 2021b). In the petal AZ of rose, RbXTH3/5/6/12 can be rapidly induced by ET within hours (Singh et al. 2013). Similarly, in citrus leaves, CitXTH1-3 levels are upregulated in the AZ after ET treatment (Agustí et al. 2009).
Apart from cell detachment, a protective layer is also formed on the surface of the distal end of the abscission layer to protect the plant from pathogen entry and water loss. Single-cell transcriptomics demonstrates that the Arabidopsis floral organ AZ is composed of two neighboring cell types with distinct cellular activities: the secession cells (SEC) of the separated organs produce a honeycomb structure of lignin, that serves as a mechanical brace to localize cell wall breakdown and spatially restrict the detachment of cells; While the residuum cells (REC) of the receptacle undergo a de novo specification of epidermal cells by recruiting two wall-hydrolyzing proteins, QRT2 and XTH28, thereby leading to the formation of a protective cuticle (Lee et al. 2018; Kim et al. 2019). Although the biochemical reactions catalyzed by these enzymes are relatively well understood, their regulation and coordination during abscission requires further investigation.
1.5 Manipulation of plant abscission in agriculture
The percentage of flower and fruitlet abscission is closely related to yield, therefore, the artificial regulation of plant abscission is an important way to ensure production. Hexanal, a natural compound produced in plants after injury, has been shown to be effective in preventing preharvest fruit drop of many fruits. For example, in apples, the application of hexanal can effectively reduce ET biosynthesis and perception in the AZ, preventing cell wall degradation, and consequently minimizing fruit drop (Sriskantharajah et al. 2021). However, in tea production, an ET-releasing molecule, ethephon, is used to stimulate flower abscission and decrease the overall number of flowers, thereby improving tea yield and quality (Zhang et al. 2022a).
Post-harvest storage and transportation of fruits and vegetables often involve issues of fruit or leaf abscission, which negatively affects their commercial value. Preharvest spraying of calcium nanoparticles on grapes results in an increased calcium pectinate content in the AZ, leading to delayed pectin degradation, suppressed ET synthesis, and inhibition of grape berry abscission (Zhu et al. 2024). Post-harvest application of nordihydroguaiaretic acid (NDGA) or chitosan can effectively inhibit the activity of cell wall-degrading enzymes, thereby delaying the cellular detachment of the AZ (Zhu et al. 2022; Wu et al. 2021). In cut flowers, petal abscission is profoundly promoted by ET, and the application of ET inhibitors such as silver thiosulfate (STS) and 1-methylcyclopropene (1-MCP) significantly extends the vase life of flowers. STS effectively reduces the activity of the enzymes involved in cell wall hydrolysis in the AZ, whereas 1-MCP inhibits ET perception by suppressing ET receptor genes and enhancing antioxidant activity (Zhang et al. 2022b; Naing et al. 2022).
Mechanical harvesting is the future of modern agriculture practices. To improve harvest productivity, it is necessary to cultivate varieties suitable for mechanical harvesting. During the processing of vegetables such as canned tomato and pepper, removing the pedicels and calyx depends on farm labor and time. Moreover, the presence of stems may cause mechanical damage to the fruits during transportation. In tomato, mutations in J, J-2, and MC disrupt AZ formation, allowing for mechanical harvesting without physical wounding during transportation (Ito and Nakano 2015). In Capsicum annuum cultivation, the Mexican landrace UCD-14 presents an easy-destemming trait, possibly due to the presence and activation of the pedicel/fruit AZ. Multiple quantitative trait locus (QTLs) known to control Arabidopsis abscission are found to co-segregate with the stem removal traits in UCD-14 (Hill et al. 2023). In Brassica napus, premature silique dehiscence results in devastating yield loss during mechanical harvesting (Li et al. 2021). Recently, modulating the expression of the hemicellulase gene BnMAN7A07 or knock-out of two IDA homologs, BnaIDA-A07 and BnaIDA-C06, suppressed silique dehiscence, improved yield, and facilitated mechanical harvesting in Brassica napus (Li et al. 2021b; Geng et al. 2022). Since rapeseed flower fields serve as significant rural tourist attractions, CRISPR-mediated BnaIDA gene editing extends the flowering period and enhances their resistance to Sclerotinia sclerotiorum, thereby boosting tourism income (Wu et al. 2022).
1.6 Future perspectives
Plant hormones play a crucial role in regulating plant abscission and their crosstalk has long been a subject of research interest. A widely acknowledged hypothesis suggests that ET promotes abscission, whereas auxins inhibit this process. For example, litchi AUXIN RESPONSE FACTOR 5 (LcARF5) and LcEIL3 are upregulated by ET and downregulated by auxins, which promote fruit abscission through the expression of LcIDL1 and LcHSL2 (Ma et al. 2024). Auxins plays various roles at different stages of abscission. A recent study indicated that JA induces autophagy to promote petal abscission (Furuta et al. 2024). In addition, gibberellins, ABA, and brassinosteroids have been reported to participate in the regulation of abscission (Marciniak et al. 2018; Wu et al. 2023a; Kućko et al. 2023; Ma et al. 2021a, b). However, the interactions between plant hormones remain unclear. Therefore, further research on the crosstalk among the hormones that participate during abscission is warranted.
1.7 Concluding remarks
In recent decades, notable progress has been made in the study of abscission in model plants, which has paved the way for translational research in crops and other non-model plants. The utilization of single-cell sequencing or spatially enhanced-resolution omics-sequencing (Stereo-seq) will enable the study of plant abscission at single-cell resolutions (Baysoy et al. 2023). Such technologies will facilitate the elucidation of heterogeneity within different AZ cell populations and provide novel insights into the developmental process of AZ, thereby establishing a genetic toolkit for the genetic manipulation of plant abscission in future agriculture. In the future, understanding the developmental timing of plant abscission will enable precise human-regulation of the process of abscission. Combined with precise plant genome editing tools (Xiong et al. 2023), it will be possible to optimize harvestability traits and design novel crop cultivars suitable for mechanized harvesting in the future.
Availability of data and materials
No datasets were generated or analyzed during the current study.
References
Agustí J, Merelo P, Cercós M, Tadeo FR, Talón M. Comparative transcriptional survey between laser-microdissected cells from laminar abscission zone and petiolar cortical tissue during ethylene-promoted abscission in citrus leaves. BMC Plant Biol. 2009;9:127.
Alam O, Purugganan MD. Domestication and the evolution of crops: variable syndromes, complex genetic architectures, and ecological entanglements. Plant Cell. 2024;36:1227–41.
Andrés F, Romera-Branchat M, Martínez-Gallegos R, Patel V, Schneeberger K, Jang S, Altmüller J, Nürnberg P, Coupland G. Floral Induction in Arabidopsis by flowering locus T Requires Direct Repression of blade-on-petiole genes by the homeodomain protein pennywise. Plant Physiol. 2015;169:2187–99.
Baysoy A, Bai Z, Satija R, Fan R. The technological landscape and applications of single-cell multi-omics. Nat Rev Mol Cell Biol. 2023;24:695–713.
Belles-Boix E, Hamant O, Witiak SM, Morin H, Traas J, Pautot V. KNAT6: an Arabidopsis homeobox gene involved in meristem activity and organ separation. Plant Cell. 2006;18:1900–7.
Burr CA, Leslie ME, Orlowski SK, Chen I, Wright CE, Daniels MJ, Liljegren SJ. Cast away, a membrane-associated receptor-like kinase, inhibits organ abscission in Arabidopsis. Plant Physiol. 2011;156:1837–50.
Butenko MA, Patterson SE, Grini PE, Stenvik GE, Amundsen SS, Mandal A, Aalen RB. Inflorescence deficient in abscission controls floral organ abscission in Arabidopsis and identifies a novel family of putative ligands in plants. Plant Cell. 2003;15:2296–307.
Butenko MA, Shi CL, Aalen RB. KNAT1, KNAT2 and KNAT6 act downstream in the IDA-HAE/HSL2 signaling pathway to regulate floral organ abscission. Plant Signaling Behav. 2012;7:135–8.
Chen MK, Hsu WH, Lee PF, Thiruvengadam M, Chen HI, Yang CH. The MADS box gene, forever young flower, acts as a repressor controlling floral organ senescence and abscission in Arabidopsis. Plant J. 2011;68:168–85.
Chen WH, Lin PT, Hsu WH, Hsu HF, Li YC, Tsao CW, Hsu MC, Mao WT, Yang CH. Regulatory network for forever young flower-like genes in regulating Arabidopsis flower senescence and abscission. Commun Biol. 2022;5:1–13.
Cho SK, Larue CT, Chevalier D, Wang HC, Jinn TL, Zhang SQ, Walker JC. Regulation of floral organ abscission in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2008;105:15629–34.
Couzigou JM, Magne K, Mondy S, Cosson V, Clements J, Ratet P. The legume NOOT-BOP-COCH-LIKE genes are conserved regulators of abscission, a major agronomical trait in cultivated crops. New Phytol. 2016;209:228–40.
Crick J, Corrigan L, Belcram K, Khan M, Dawson JW, Adroher B, Li SB, Hepworth SR, Pautot V. Floral organ abscission in Arabidopsis requires the combined activities of three tale homeodomain transcription factors. J Exp Bot. 2022;73:6150–69.
Damodharan S, Zhao DZ, Arazi T. A common miRNA160-based mechanism regulates ovary patterning, floral organ abscission and lamina outgrowth in tomato. Plant J. 2016;86:458–71.
Del Campillo E, Bennett AB. Pedicel breakstrength and cellulase gene expression during tomato flower abscission. Plant Physiol. 1996;111:813–20.
Dong XF, Liu XF, Cheng LN, Li RZ, Ge SQ, Wang S, Cai Y, Liu Y, Meng SD, Jiang CZ, Shi CL, Li TL, Fu DQ, Qi MF, Xu T. SlBEL11 regulates flavonoid biosynthesis, thus fine-tuning auxin efflux to prevent premature fruit drop in tomato. J Integr Plant Biol. 2024;66:749–70.
Dutta SK, Gurung G, Yadav A, Laha R, Mishra VK. Factors associated with citrus fruit abscission and management strategies developed so far: a review. New Zeal J Crop Hort. 2023;51:467–88.
Estornell LH, Agustí J, Merelo P, Talón M, Tadeo FR. Elucidating mechanisms underlying organ abscission. Plant Sci. 2013;199–200:48–60.
Estornell LH, Wildhagen M, Perez-Amador MA, Talon M, Tadeo FR, Butenko MA. The IDA peptide controls abscission in Arabidopsis and citrus. Front Plant Sci. 2015;6:1003.
Ferrándiz C. Regulation of fruit dehiscence in Arabidopsis. J Exp Bot. 2002;53:2031–8.
Furuta Y, Yamamoto H, Hirakawa T, Uemura A, Pelayo MA, Iimura H, Katagiri N, Takeda-Kamiya N, Kumaishi K, Shirakawa M, Ishiguro S, Ichihashi Y, Suzuki T, Goh T, Toyooka K, Ito T, Yamaguchi N. Petal abscission is promoted by jasmonic acid-induced autophagy at arabidopsis petal bases. Nat Commun. 2024;15:1098.
Galindo-Trigo S, Khandare V, Roosjen M, Adams J, Wangler AM, Bayer M, Borst JW, Smakowska-Luzan E, Butenko MA. A multifaceted kinase axis regulates plant organ abscission through conserved signaling mechanisms. Curr Biol. 2024a;34:3020–30.
Galindo-Trigo S, Bågman AM, Ishida T, Sawa S, Brady SM, Butenko MA. Dissection of the IDA promoter identifies WRKY transcription factors as abscission regulators in Arabidopsis. J Exp Bot. 2024b;75:2417–34.
Geng R, Shan Y, Li L, Shi CL, Zhang W, Wang J, Sarwar R, Xue YX, Li YL, Zhu KM, Wang Z, Xu LZ, Aalen RB, Tan XL. CRISPR-mediated BnaIDA editing prevents silique shattering, floral organ abscission, and spreading of Sclerotinia sclerotiorum in brassica napus. Plant Commun. 2022;3:100452.
Gomez-Mena C, Sablowski R. Arabidopsis thaliana homeobox gene1 establishes the basal boundaries of shoot organs and controls stem growth. Plant cell. 2008;20:2059–72.
González-Carranza ZH, Rompa U, Peters JL, Bhatt AM, Wagstaff C, Stead AD, Roberts JA. Hawaiian skirt: an F-box gene that regulates organ fusion and growth in arabidopsis. Plant Physiol. 2007;144:1370–82.
Goto K, Yabuta S, Tamaru S, Ssenyonga P, Emanuel B, Katsuhama N, Sakagami JI. Root hypoxia causes oxidative damage on photosynthetic apparatus and interacts with light stress to trigger abscission of lower position leaves in Capsicum. Sci Hortic. 2022;305:111337.
Gubert CM, Liljegren SJ. Haesa and Haesa-like2 activate organ abscission downstream of NEVERSHED and EVERSHED in Arabidopsis flowers. Plant Signaling Behav. 2014a;9:e29115.
Gubert CM, Christy ME, Ward DL, Groner WD, Liljegren SJ. ASYMMETRIC LEAVES1 regulates abscission zone placement in Arabidopsis flowers. BMC Plant Biol. 2014b;14:195.
Guo M, Thomas J, Collins G, Timmermans MCP. Direct repression of knox loci by the asymmetric leaves1 Complex of Arabidopsis. Plant Cell. 2008;20:48–58.
He HK, Bai M, Tong PP, Hu YT, Yang M, Wu H. Cellulase6 and Mannanase7 affect cell differentiation and silique dehiscence. Plant Physiol. 2018;176:2186–201.
Hepworth SR, Zhang YL, McKim S, Li X, Haughn GW. BLADE-ON-PETIOLE–Dependent Signaling Controls Leaf and Floral Patterning in Arabidopsis. Plant Cell. 2005;17:1434–48.
Hill T, Cassibba V, Joukhadar I, Tonnessen B, Havlik C, Ortega F, Sripolcharoen S, Visser BJ, Stoffel K, Thammapichai P, Garcia-Llanos A, Chen SY, Hulse-Kemp A, Walker S, Van Deynze A. Genetics of destemming in pepper: A step towards mechanical harvesting. Front Genet. 2023;14:1114832.
Inoue C, Htun TM, Inoue K, Ikeda K, Ishii T, Ishikawa R. Inhibition of abscission layer formation by an interaction of two seed-shattering loci, sh4 and qSH3, in rice. Genes Genet Syst. 2015;90:1–9.
Ito Y, Nakano T. Development and regulation of pedicel abscission in tomato. Front Plant Sci. 2015;6:422.
Ji H, Kim SR, Kim YH, Kim H, Eun MY, Jin ID, Cha YS, Yun DW, Ahn BO, Lee MC, Lee GS, Yoon UH, Lee JS, Lee YH, Suh SC, Jiang WZ, Yang JH, Jin P, McCouch SR, An G, Koh HJ. Inactivation of the CTD phosphatase-like gene OsCPL1 enhances the development of the abscission layer and seed shattering in rice. Plant J. 2010;61:96–106.
Jiang LY, Ma X, Zhao SS, Tang YY, Liu FX, Gu P, Fu YC, Zhu ZF, Cai HW, Sun CQ, Tan LB. The APETALA2-Like Transcription Factor SUPERNUMERARY BRACT Controls Rice Seed Shattering and Seed Size. Plant Cell. 2019;31:17–36.
Jinn TL, Stone JM, Walker JC. HAESA, an Arabidopsis leucine-rich repeat receptor kinase, controls floral organ abscission. Genes Dev. 2000;14:108–17.
Jun JH, Ha CM, Fletcher JC. Blade-on-petiole1 coordinates organ determinacy and axial polarity in Arabidopsis by directly activating asymmetric leaves2. Plant Cell. 2010;22:62–76.
Kalaitzis P, Solomos T, Tucker ML. Three different polygalacturonases are expressed in tomato leaf and flower abscission, each with a different temporal expression pattern. Plant Physiol. 1997;113:1303–8.
Khan M, Ragni L, Tabb P, Salasini BC, Chatfield S, Datla R, Lock J, Kuai X, Després C, Proveniers M, Yongguo C, Xiang D, Morin H, Rullière JP, Citerne S, Hepworth SR, Pautot V. Repression of lateral organ boundary genes by pennywise and pound-foolish is essential for meristem maintenance and flowering in Arabidopsis. Plant Physiol. 2015;169:2166–86.
Kim J, Chun JP, Tucker ML. Transcriptional regulation of abscission zones. Plants. 2019;8:154.
Kon TM, Clavet CD, Clarke GG. Organic aminoethoxyvinylglycine is an effective alternative for reducing apple preharvest drop. HortScience. 2023;58:733–8.
Konishi S, Izawa T, Lin SY, Ebana K, Fukuta Y, Sasaki T, Yano M. An SNP caused loss of seed shattering during rice domestication. Science. 2006;312:1392–6.
Kućko A, de Dios Alché J, Tranbarger TJ, Wilmowicz E. Abscisic acid- and ethylene-induced abscission of yellow lupine flowers is mediated by jasmonates. J Plant Physiol. 2023;290:154119.
Lashbrook CC, Cai SQ. Cell wall remodeling in Arabidopsis stamen abscission zones. Plant Signaling Behav. 2008;3:733–6.
Lee Y, Yoon TH, Lee J, Jeon SY, Lee JH, Lee MK, Chen HZ, Yun J, Oh SY, Wen XH, Cho HK, Mang H, Kwak JM. A Lignin molecular brace controls precision processing of cell walls critical for surface integrity in Arabidopsis. Cell. 2018;173:1468–80.
Leslie ME, Lewis MW, Youn JY, Daniels MJ, Liljegren SJ. The evershed receptor-like kinase modulates floral organ shedding in Arabidopsis. Development. 2010;137:467–76.
Li CB, Zhou AL, Sang T. Rice domestication by reducing shattering. Science. 2006;311:1936–9.
Li CQ, Zhao ML, Ma XS, Wen ZX, Ying PY, Peng MJ, Ning XP, Xia R, Wu H, Li JG. The HD-Zip transcription factor LcHB2 regulates litchi fruit abscission through the activation of two cellulase genes. J Exp Bot. 2019;70:5189–203.
Li J, Chen G, Zhang J, Shen H, Kang J, Feng P, Xie Q, Hu Z. Suppression of a hexokinase gene, SlHXK1, leads to accelerated leaf senescence and stunted plant growth in tomato. Plant Sci. 2020;298:110544.
Li RZ, Shi CL, Wang XY, Meng Y, Cheng L, Jiang CZ, Qi MF, Xu T, Li TL. Inflorescence abscission protein SlIDL6 promotes low light intensity-induced tomato flower abscission. Plant Physiol. 2021;186:1288–301.
Li YL, Yu YK, Zhu KM, Ding LN, Wang Z, Yang YH, Cao J, Xu LZ, Li YM, Tan XL. Down-regulation of MANNANASE7 gene in Brassica napus L. enhances silique dehiscence-resistance. Plant Cell Rep. 2021b;40:361–74.
Liljegren SJ, Leslie ME, Darnielle L, Lewis MW, Taylor SM, Luo R, Geldner N, Chory J, Randazzo PA, Yanofsky MF, Ecker JR. Regulation of membrane trafficking and organ separation by the NEVERSHED ARF-GAP protein. Development. 2009;136:1909–18.
Lin ZW, Griffith ME, Li XR, Zhu ZF, Tan LB, Fu YC, Zhang WX, Wang XK, Xie DX, Sun CQ. Origin of seed shattering in rice (Oryza sativa L.). Planta. 2007;226:11–20.
Lin ZW, Li XR, Shannon LM, Yeh CT, Wang ML, Bai GH, Peng Z, Li JR, Trick HN, Clemente TE, Doebley J, Schnable PS, Tuinstra MR, Tesso TT, White F, Yu J. Parallel domestication of the Shattering1 genes in cereals. Nat Genet. 2012;44:720–4.
Liu DM, Wang D, Qin ZR, Zhang DD, Yin LJ, Wu L, Colasanti J, Li A, Mao L. The sepallata mads-box protein slmbp21 forms protein complexes with jointless and macrocalyx as a transcription activator for development of the tomato flower abscission zone. Plant J. 2014;77:284–96.
Liu XF, Cheng LN, Li RZ, Cai Y, Wang XY, Fu X, Dong XF, Qi MF, Jiang CZ, Xu T, Li TL. The HD-Zip transcription factor SlHB15A regulates abscission by modulating jasmonoyl-isoleucine biosynthesis. Plant Physiol. 2022;189:2396–412.
Lu L, Arif S, Yu JM, Lee JW, Park YH, Tucker ML, Kim J. Involvement of IDA-HAE module in natural development of tomato flower abscission. Plants. 2023;12:185.
Lv SW, Wu WG, Wang MH, Meyer RS, Ndjiondjop M-N, Tan BL, Zhou HY, Zhang JW, Fu YC, Cai HW, Sun CQ, Wing RA, Zhu ZF. Genetic control of seed shattering during African rice domestication. Nat Plants. 2018;4:331–7.
Ma C, Meir S, Xiao LT, Tong JH, Liu Q, Reid MS, Jiang CZ. A KNOTTED1-LIKE HOMEOBOX Protein Regulates Abscission in Tomato by Modulating the Auxin Pathway. Plant Physiol. 2015;167:844–53.
Ma XS, Yuan Y, Li CQ, Wu Q, He ZD, Li JG, Zhao ML. Brassinosteroids suppress ethylene-induced fruitlet abscission through LcBZR1/2-mediated transcriptional repression of LcACS1/4 and LcACO2/3 in litchi. Hort Res. 2021;8:105.
Ma XS, Li CQ, Yuan Y, Zhao ML, Li JG. Xyloglucan endotransglucosylase/hydrolase genes LcXTH4/7/19 are involved in fruitlet abscission and are activated by LcEIL2/3 in litchi. Physiol Plant. 2021;173:1136–46.
Ma XS, He ZD, Yuan Y, Liang ZJ, Zhang H, Lalun VO, Liu ZY, Zhang YQ, Huang ZQ, Huang YL, Li JG, Zhao ML. The transcriptional control of LcIDL1–LcHSL2 complex by LcARF5 integrates auxin and ethylene signaling for litchi fruitlet abscission. J Integr Plant Biol. 2024;66:1206–26.
Marciniak K, Kućko A, Wilmowicz E, Świdziński M, Przedniczek K, Kopcewicz J. Gibberellic acid affects the functioning of the flower abscission zone in Lupinus luteus via cooperation with the ethylene precursor independently of abscisic acid. J Plant Physiol. 2018;229:170–4.
McKim SM, Stenvik G-E, Butenko MA, Kristiansen W, Cho SK, Hepworth SR, Aalen RB, Haughn GW. The blade-on-petiole genes are essential for abscission zone formation in Arabidopsis. Development. 2008;135:1537–46.
Meir S, Philosoph-Hadas S, Riov J, Tucker ML, Patterson SE, Roberts JA. Re-evaluation of the ethylene-dependent and -independent pathways in the regulation of floral and organ abscission. J Exp Bot. 2019;70:1461–7.
Meng XZ, Zhou GJ, Tang J, Li B, de Oliveira MVV, Chai JJ, He P, Shan LB. Ligand-induced receptor-like kinase complex regulates floral organ abscission in Arabidopsis. Cell Rep. 2016;14:1330–8.
Meng SD, Xiang HZ, Yang XR, Ye YZ, Han LL, Xu T, Liu YF, Wang F, Tan CH, Qi MF, Li TL. Effects of low temperature on pedicel abscission and auxin synthesis key genes of tomato. Int J Mol Sci. 2023;24:9186.
Naing AH, Win NM, Kyu SY, Kang IK, Kim CK. Current progress in application of 1-Methylcyclopropene to improve postharvest quality of cut flowers. Hortic Plant J. 2022;8:676–88.
Nakano T, Kimbara J, Fujisawa M, Kitagawa M, Ihashi N, Maeda H, Kasumi T, Ito Y. MACROCALYX and JOINTLESS Interact in the Transcriptional Regulation of Tomato Fruit Abscission Zone Development. Plant Physiol. 2012;158:439–50.
Nakano T, Fujisawa M, Shima Y, Ito Y. Expression profiling of tomato pre-abscission pedicels provides insights into abscission zone properties including competence to respond to abscission signals. BMC Plant Biol. 2013;13:40.
Nakano T, Fujisawa M, Shima Y, Ito Y. The AP2/ERF transcription factor SlERF52 functions in flower pedicel abscission in tomato. J Exp Bot. 2014;65:3111–9.
Ning J, He W, Wu LH, Chang LQ, Hu M, Fu YC, Liu FX, Sun HY, Gu P, Ndjiondjop M, Sun CQ, Zhu ZF. The MYB transcription factor Seed Shattering 11 controls seed shattering by repressing lignin synthesis in African rice. Plant Biotechnol J. 2023;21:931–42.
Nunes AL, Delatorre CA, Merotto A Jr. Gene expression related to seed shattering and the cell wall in cultivated and weedy rice. Plant Biol. 2014;16:888–96.
Ogawa M, Kay P, Wilson S, Swain SM. Arabidopsis dehiscence zone polygalacturonase1 (ADPG1), ADPG2, and QUARTET2 are polygalacturonases required for cell separation during reproductive development in Arabidopsis. Plant Cell. 2009;21:216–33.
Patharkar OR, Walker JC. Floral organ abscission is regulated by a positive feedback loop. Proc Natl Acad Sci USA. 2015;112:2906–11.
Patharkar OR, Walker JC. Core Mechanisms Regulating Developmentally Timed and Environmentally Triggered Abscission. Plant Physiol. 2016;172:510–20.
Patharkar OR, Gassmann W, Walker JC. Leaf shedding as an anti-bacterial defense in Arabidopsis cauline leaves. PLoS Genet. 2017;13:e1007132.
Patterson SE. Cutting loose. Abscission and dehiscence in Arabidopsis. Plant Physiol. 2001;126:494–500.
Qiu ZL, Wen Z, Hou Q, Qiao GD, Yang K, Hong Y, Wen XP. Cross-talk between transcriptome, phytohormone and HD-ZIP gene family analysis illuminates the molecular mechanism underlying fruitlet abscission in sweet cherry (Prunus avium L). BMC Plant Biol. 2021;21:173.
Ragni L, Belles-Boix E, Gunl M, Pautot V. Interaction of KNAT6 and KNAT2 with BREVIPEDICELLUS and PENNYWISE in Arabidopsis inflorescences. Plant Cell. 2008;20:888–900.
Reichardt S, Piepho HP, Stintzi A, Schaller A. Peptide signaling for drought-induced tomato flower drop. Science. 2020;367:1482–5.
Roberts JA, Schindler CB, Tucker GA. Ethylene-promoted tomato flower abscission and the possible involvement of an inhibitor. Planta. 1984;160:159–63.
Roldan MVG, Périlleux C, Morin H, Huerga-Fernandez S, Latrasse D, Benhamed M, Bendahmane A. Natural and induced loss of function mutations in SlMBP21 MADS-box gene led to jointless-2 phenotype in tomato. Sci Rep. 2017;7:4402.
Ruiz R, García-Luis A, Monerri C, Guardiola JL. Carbohydrate availability in relation to fruitlet abscission in Citrus. Ann Bot. 2001;87:805–12.
Schippers P, Jongejans E. Release thresholds strongly determine the range of seed dispersal by wind. Ecol Model. 2005;185:93–103.
Sexton R, Roberts JA. Cell Biology of Abscission. Ann Rev Plant Physio. 1982;33:133–62.
Shi CL, Stenvik GE, Vie AK, Bones AM, Pautot V, Proveniers M, Aalen RB, Butenko MA. Arabidopsis Class I KNOTTED-Like Homeobox Proteins Act Downstream in the IDA-HAE/HSL2 Floral Abscission Signaling Pathway. Plant Cell. 2011;23:2553–67.
Shi ZH, Jiang Y, Han XQ, Liu X, Cao RS, Qi MF, Xu T, Li TL. SlPIN1 regulates auxin efflux to affect flower abscission process. Sci Rep. 2017;7:14919.
Singh AP, Dubey S, Lakhwani D, Pandey SP, Khan K, Dwivedi UN, Nath P, Sane AP. Differential expression of several xyloglucan endotransglucosylase/hydrolase genes regulates flower opening and petal abscission in roses. AoB Plants. 2013;5:plt030.
Singh P, Maurya SK, Singh D, Sane AP. The rose inflorescence deficient in abscission-like genes, RbIDL1 and RbIDL4, regulate abscission in an ethylene-responsive manner. Plant Cell Rep. 2023;42:1147–61.
Song SK, Yun YB, Lee MM. Shoot meristemless is required for the proper internode patterning and the sepal separation in Arabidopsis. J Plant Biol. 2020;63:33–42.
Sriskantharajah K, El Kayal W, Torkamaneh D, Ayyanath MM, Saxena PK, Sullivan AJ, Paliyath G, Subramanian J. Transcriptomics of improved fruit retention by hexanal in ‘honeycrisp’ reveals hormonal crosstalk and reduced cell wall degradation in the fruit abscission zone. Int J Mol Sci. 2021;22:8830.
Stenvik GE, Tandstad NM, Guo YF, Shi CL, Kristiansen W, Holmgren A, Clark SE, Aalen RB, Butenko MA. The EPIP peptide of inflorescence deficient in abscission is sufficient to induce abscission in Arabidopsis through the receptor-like kinases HAESA and HAESA-LIKE2. Plant Cell. 2008;20:1805–17.
Su SH, Lei YW, Zhou X, Suzuki T, Xiao W, Higashiyama T. A blade-on-petiole orthologue regulates corolla differentiation in the proximal region in Torenia fournieri. Nat Commun. 2023;14:4763.
Sun PY, Zhang WH, Wang YH, He Q, Shu F, Liu H, Wang J, Wang JM, Yuan LP, Deng HF. OsGRF4 controls grain shape, panicle length and seed shattering in rice. J Integr Plant Biol. 2016;58:836–47.
Taylor JE, Whitelaw CA. Signals in abscission. New Phytol. 2001;151:323–40.
Taylor I, Baer J, Calcutt R, Walker JC. Hypermorphic SERK1 mutations function via a SOBIR1 pathway to activate floral abscission signaling. Plant Physiol. 2019;180:1219–29.
Tsuchiya M, Satoh S, Iwai H. Distribution of XTH, expansin, and secondary-wall-related CesA in floral and fruit abscission zones during fruit development in tomato (Solanum lycopersicum). Front Plant Sci. 2015;6:323.
Tsujimura Y, Sugiyama S, Otsuka K, Htun TM, Numaguchi K, Castillo C, Akagi T, Ishii T, Ishikawa R. Detection of a novel locus involved in non-seed-shattering behaviour of Japonica rice cultivar, Oryza sativa ‘Nipponbare.’ Theor Appl Genet. 2019;132:2615–23.
Tucker ML, Yang R. IDA-like gene expression in soybean and tomato leaf abscission and requirement for a diffusible stelar abscission signal. AoB Plants. 2012;2012:pls035.
Tucker ML, Burke A, Murphy CA, Thai VK, Ehrenfried ML. Gene expression profiles for cell wall-modifying proteins associated with soybean cyst nematode infection, petiole abscission, root tips, flowers, apical buds, and leaves. J Exp Bot. 2007;58:3395–406.
Ventimilla D, Velázquez K, Ruiz-Ruiz S, Terol J, Pérez-Amador MA, Vives MC, Guerri J, Talon M, Tadeo FR. IDA (Inflorescence deficient in abscission)-like peptides and HAE (HAESA)-like receptors regulate corolla abscission in Nicotiana benthamiana flowers. BMC Plant Biol. 2021;21:226.
Villalobos-Vega R, Goldstein G, Haridasan M, Franco AC, Miralles-Wilhelm F, Scholz FG, Bucci SJ. Leaf litter manipulations alter soil physicochemical properties and tree growth in a Neotropical savanna. Plant Soil. 2011;346:385–97.
Wang GQ, Wei PC, Tan F, Yu M, Zhang XY, Chen QJ, Wang XC. The transcription factor AtDOF4 is involved in ethylene- and IDA-mediated organ abscission in Arabidopsis. Front Plant Sci. 2016;7:184083.
Wang F, Zheng ZH, Yuan Y, Li JG, Zhao ML. Identification and characterization of haesa-like genes involved in the fruitlet abscission in Litchi. Int J Mol Sci. 2019;20:5945.
Wang Y, Salasini BC, Khan M, Devi B, Bush M, Subramaniam R, Hepworth SR. Clade I TGACG-motif binding basic leucine zipper transcription factors mediate blade-on-petiole-dependent regulation of development. Plant Physiol. 2019;180:937–51.
Webster AD. Factors influencing the flowering, fruit set and fruit growth of european pears. Acta Hortic. 2002;699–709. https://doi.org/10.17660/ActaHortic.2002.596.121.
Wei PC, Tan F, Gao XQ, Zhang XQ, Wang GQ, Xu H, Li LJ, Chen J, Wang XC. Overexpression of AtDOF4.7, an Arabidopsis DOF family transcription factor, induces floral organ abscission deficiency in Arabidopsis. Plant Physiol. 2010;153:1031–45.
Wu XM, Yu Y, Han LB, Li CL, Wang HY, Zhong NQ, Yao Y, Xia GX. The tobacco blade-on-petiole2 gene mediates differentiation of the corolla abscission zone by controlling longitudinal cell expansion. Plant Physiol. 2012;159:835–50.
Wu PW, Xin FY, Xu HJL, Chu YY, Du YL, Tian HQ, Zhu BZ. Chitosan inhibits postharvest berry abscission of ‘Kyoho’ table grapes by affecting the structure of abscission zone, cell wall degrading enzymes and SO2 permeation. Postharvest Biol Technol. 2021;176:111507.
Wu J, Liu HM, Ren SC, Li PP, Li X, Lin L, Sun QF, Zhang L, Lin C, Wang YP. Generating an oilseed rape mutant with non-abscising floral organs using CRISPR/Cas9 technology. Plant Physiol. 2022;190:1562–5.
Wu H, He Q, He B, He SY, Zeng LJ, Yang LB, Zhang H, Wei ZR, Hu XM, Hu J, Zhang Y, Shang LG, Wang SK, Cui P, Xiong GS, Qian Q, Wang Q. Gibberellin signaling regulates lignin biosynthesis to modulate rice seed shattering. Plant Cell. 2023a;35:4383–404.
Wu H, He Q, Wang Q. Advances in rice seed shattering. Int J Mol Sci. 2023b;24:8889.
Wu GY, Li QY, Tan Y, Wang S, Liu YY, Liu YL. Advances in understanding the mechanisms of organ abscission in vivo and in vitro plants. Plant Growth Regul. 2024;103:293–306.
Xie QL, Hu ZL, Zhu ZG, Dong TT, Zhao ZP, Cui B, Chen GP. Overexpression of a novel MADS-box gene SlFYFL delays senescence, fruit ripening and abscission in tomato. Sci Rep. 2014;4:4367.
Xie YN, Yang T, Zhang BT, Qi QQ, Ding AM, Shang LG, Zhang Y, Qian Q, Zhang ZF, Yan N. Systematic Analysis of BELL Family Genes in Zizania latifolia and Functional Identification of ZlqSH1a/b in Rice Seed Shattering. Int J Mol Sci. 2022;23:15939.
Xiong XY, Liu KH, Li ZX, Xia FN, Ruan XM, He XL, Li JF. Split complementation of base editors to minimize off-target edits. Nat Plants. 2023;9:1832–47.
Xu C, Park SJ, Van Eck J, Lippman ZB. Control of inflorescence architecture in tomato by BTB/POZ transcriptional regulators. Genes Dev. 2016;30:2048–61.
Xu PP, Chen HY, Cai WM. Transcription factor CDF4 promotes leaf senescence and floral organ abscission by regulating abscisic acid and reactive oxygen species pathways in Arabidopsis. Embo Rep. 2020;21:e48967.
Yan F, Gong ZH, Hu GJ, Ma XS, Bai RY, Yu RN, Zhang Q, Deng W, Li ZG, Wuriyanghan H. Tomato SlBL4 plays an important role in fruit pedicel organogenesis and abscission. Hortic. 2021;8:78.
Ying PY, Li CQ, Liu XC, Xia R, Zhao ML, Li JG. Identification and molecular characterization of an IDA-like gene from litchi, LcIDL1, whose ectopic expression promotes floral organ abscission in Arabidopsis. Sci Rep. 2016;6:37135.
Yoon J, Cho LH, Kim SL, Choi H, Koh HJ, An G. The BEL1-type homeobox gene SH5 induces seed shattering by enhancing abscission-zone development and inhibiting lignin biosynthesis. Plant J. 2014;79:717–28.
Yoon J, Cho LH, Antt HW, Koh HJ, An G. KNOX Protein OSH15 Induces Grain Shattering by Repressing Lignin Biosynthesis Genes. Plant Physiol. 2017;174:312–25.
Yu YQ, Kellogg EA. Multifaceted mechanisms controlling grain disarticulation in the Poaceae. Curr Opin Plant Biol. 2024;81:102564.
Zhang LG, Sun LL, Zhang XF, Zhang SQ, Xie DW, Liang CB, Huang WG, Fan LJ, Fang YY, Chang Y. OFP1 Interaction with ATH1 regulates stem growth, flowering time and flower basal boundary formation in Arabidopsis. Genes. 2018;9:399.
Zhang JJ, Zhang YY, He YM, Du TT, Shan DX, Fan HD, Wang WY, Qin Z, Xin CH, Pei HX. Metabolome and transcriptome integration reveals insights into the process of delayed petal abscission in rose by STS. Front Plant Sci. 2022;13:1045270.
Zhang XZ, Li BQ, Zhang XZ, Wang C, Zhang ZQ, Sun P. Exogenous application of ethephon regulates flower abscission, shoot growth, and secondary metabolites in Camellia sinensis. Sci Hortic-amsterdam. 2022;304:111333.
Zhao ML, Li JG. Molecular events involved in fruitlet abscission in Litchi. Plants. 2020;9:151.
Zhao ML, Li CQ, Ma XS, Xia R, Chen JY, Liu XC, Ying PY, Peng MJ, Wang J, Shi CL, Li JG. KNOX protein KNAT1 regulates fruitlet abscission in litchi by repressing ethylene biosynthetic genes. J Exp Bot. 2020;71:4069–82.
Zhou Y, Lu DF, Li CY, Luo JH, Zhu BF, Zhu JJ, Shangguan YY, Wang ZX, Sang T, Zhou B, Han B. Genetic control of seed shattering in rice by the APETALA2 transcription factor shattering abortion1. Plant Cell. 2012;24:1034–48.
Zhou GY, Xu S, Ciais P, Manzoni S, Fang JY, Yu RR, Tang XL, Zhou P, Wang WT, Yan JH, Wang GX, Ma K, Li SG, Du S, Han SJ, Ma YX, Zhang DQ, Liu JX, Liu SZ, Chu GW, Zhang QM, Li YL, Huang WJ, Ren H, Lu XK, Chen XZ. Climate and litter C/N ratio constrain soil organic carbon accumulation. Natl Sci Rev. 2019;6:746–57.
Zhu MT, Liu ZF, Zeng YQ, Yu J. Nordihydroguaiaretic acid reduces postharvest berry abscission in grapes. Postharvest Biol Tec. 2022;183:111748.
Zhu MT, Li J, Liu Y, Wang QY, Fan ZW, Zeng JY, Yu J. Preharvest Nano-calcium Reduces the Table Grape Berry Abscission by Regulating Ethylene Production During Storage. J Plant Growth Regul. 2024;43:1400–9.
Acknowledgement
We thank all the members of the SU Lab.
Funding
This work was funded by the National Natural Science Foundation of China (No. 32302590), and Guangdong Basic and Applied Basic Research Foundation (2024A1515011175).
Author information
Authors and Affiliations
Contributions
S.S. and J.L. conceived the study conception and design. J.L. wrote the first version of manuscript. S. S. reviewed and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors approved the final manuscript and the submission to this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, J., Su, S. Abscission in plants: from mechanism to applications. Adv. Biotechnol. 2, 27 (2024). https://doi.org/10.1007/s44307-024-00033-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44307-024-00033-9