Abstract
The cholesterol detection of human blood using a two-dimensional photonic crystal biosensor has been studied in this paper. The structure calculations are done using Plane Wave Expansion (PWE) and Finite Difference Time Domain (FDTD). Photonic crystals can be used to detect biological components by utilizing a two-dimensional structure and resonator nano ring. These nano rings cause waveguide and resonator coupling. The cylinders around the sensing region have investigated the improvement structure output. The quality factor and figure of merit (FOM) for this sensor are 4988 and 57.14 RIU−1, respectively, and the low detection limit (DL) is 0.001(RIU), with a sensitivity of 32 (nm/RIU).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The body naturally produces cholesterol, which is one of the biomolecules, a steroid found in lipids, and a form of alcohol known as a sterol. Cholesterol is a key component of membranes and a type of fat. Blood also contains cholesterol, which comes from two main sources: diet (25%) and liver (75%) respectively. Foods with high cholesterol can refer to meat, liver, brain, eggs, and dairy products; in contrast, plant foods have no cholesterol [1,2,3].
Following a meal, cholesterol is absorbed by the intestine and then enclosed in a protein coating together with triglycerides. This mixture of proteins and fats is known as a chylomicron [4,5,6]. The liver can both create and eliminate cholesterol from the blood [7,8,9]. The growth of arterial plaques and atherosclerosis development can be influenced by blood cholesterol levels. These plaques build up on the blood vessel walls, especially the coronary vessels, causing them to narrow. This narrowing ultimately reduces blood flow, plugs the vessels, and results in heart attacks. A 4-ring molecule containing 27 carbon atoms makes up cholesterol. This lipid compound has a steroid structure created by four hydrocarbon rings. A hydrocarbon tail is attached to one end of the cholesterol molecule, and a steroid and a hydroxyl group are attached to the other. The hydroxyl group can form a hydrogen bond with the carbonyl oxygen in phospholipids and sphingolipids. Due to its composition of steroids and alcohol, cholesterol falls within the category of sterols [10,11,12].
High-density lipoprotein (HDL): The HDL carries cholesterol from the cell to the liver or carries it outside the body as waste. Because of this, HDL is referred to as "good cholesterol," and the more HDL present, the better.
Low-density lipoprotein (LDL): The cells that require cholesterol are transported there by it. If there is more cholesterol available than what is needed by the cells, excess cholesterol is deposited in the walls of the arteries, which leads to artery-related disorders. LDL is known as harmful cholesterol for this reason. The LDL-blocked arteries are depicted in Fig. 1a [13,14,15].
According to the Table 1, a normal 70 kg person has 140 g of cholesterol in their body.
The envisioned structure comprises dielectric rods within an air medium, featuring a resonator nanoring positioned centrally amidst two input and output waveguides. This nanoring serves as the focal point for sensing, with specific rods designed as cylinders or tubes to enable biomolecule attachment. Subsequent iterations involved increasing the diameter of these measuring cylinders, thereby influencing analyte-light interaction and subsequently augmenting the sensor's quality factor and sensitivity. Concurrently, densification of the nanoring's outer wall was achieved by augmenting rod numbers, thereby enhancing light confinement and interaction with target biomolecules. The entrance of the nanoring is adorned with three rows of dielectric rods, where a higher count of these coupled rods correlates with an increased sensor quality factor albeit at the expense of reduced incoming light intensity. To mitigate this intensity reduction, the radius of the third coupled rod was decreased.
1.1 Photonic crystals
By periodically altering the insulating material's refractive index or by forming a regular row of holes or cylinders, a photonic crystal can be made [16,17,18,19,20]. The photonic crystals are alternative micro-nano-structures of dielectric/metal-dielectric, which influence the pathway of the electromagnetic wave. This reflection happens over a wide spectrum of wavelengths. In other words, the photonic band gap (PBG), the foundation of operation in photonic crystals, is the wavelength or frequency of light that cannot pass through the material [21,22,23].
Photonic crystals can confine light better than other optical structures, and this is one of the suitable properties for making photonic crystal sensors. It is one of the photonic crystal structures in the design of biosensors such as blood component detection sensors that can detect blood parameters [24,25,26,27,28,29,30,31,32,33]. Another biosensor created with a photonic crystal structure is the detection of different types of cancer cells [34,35,36] and potential malaria detection [37,38,39,40,41]. Another application of photonic crystals, Salinity, and temperature detection for seawater [42,43,44]. Photonic crystals can also be used to detect chemical gases and dangerous toxins [45,46,47].
2 Materials and methods
Maxwell equations should be solved assumptions of an isotropic, non-magnetic, and charge-free medium to study the propagation of EM waves in a 2D periodic medium [48,49,50,51,52,53].
where \({\varepsilon }_{r}\) is the relative permittivity of the material and \({\varepsilon }_{0}\) and \({\mu }_{0}\) are the permeability and permittivity of the free space, respectively.
In these equations, E and H are respectively the electrical and magnetic field intensity, additionally, D and B are respectively the electrical and magnetic flux density. \({j}_{e}\) electric current source and \({j}_{m}\) electromagnetic current source.
The following are the two curl formulas for harmonic waves moving through periodic media at frequency \(\omega\):
The finite-difference time-domain (FDTD) approach can be used to determine the electromagnetic fields inside Photonic Crystals [54,55,56]. This method uses the FDTD updating equation to calculate the electromagnetic field within the photonic crystal at every instant of time using the field's past and current values [57,58,59]. The FDTD updating equations' source-free Maxwell curl equation in the time domain is:
is calculated by applying the central difference to the Eqs. (1)–(2). In this case, the integer indexes \(m\), \(n\), and \(p\) are connected to the \(x\), \(y\), and \(t\), respectively. The following are the pertinent FDTD updating equations:
FullWave offers a versatile simulation package designed to analyze the propagation of light waves in diverse geometries. It relies on the finite-difference time-domain (FDTD) technique, a well-established method for solving Maxwell's equations without approximations or theoretical limitations. Widely utilized in integrated optics, FDTD excels in scenarios where alternative methods like the Beam Propagation Method (BPM) struggle with complex geometries or fail to provide satisfactory solutions. By directly solving Maxwell’s curl equations, FDTD captures a broader range of effects compared to approximate methods. Imagine a region of space which contains no flowing currents or isolated charges. Maxwell's curl equations can be written in Cartesian coordinates as six simple scalar equations [71,72,73,74]:
The remaining four are analogous counterparts derived by cyclically interchanging the subscripts and derivatives of x, y, and z. Maxwell’s equations depict a scenario where the evolution of the electric field (E) over time is linked to the spatial fluctuations of the magnetic field (H), and conversely.
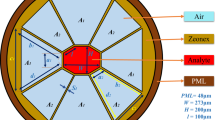
The boundary conditions at the spatial boundaries of the computational domain require meticulous attention. Numerous simulations utilize absorbing boundary conditions to remove outward-propagating energy reaching the domain boundaries. Among the most efficient methods is the perfectly matched layer (PML), where electric and magnetic conductivities are introduced to maintain a constant wave impedance, effectively absorbing energy without causing reflections. The use of the perfectly matched layer (PML) technique is being considered for boundary settings along the structure's edges. Employing PML enables enhanced absorption of electrical and magnetic energy, minimizing reflections [76,77,78].
In this structure PML width is 0.5 µm and boundary-max, boundary-min are 7.2 and −7.2 Respectively.
There are two methods used by photonic crystal sensors' measurement mechanisms:
-
Resonance wavelength shift measurement
-
Determining the transmission spectrum's intensity at its resonant wavelength
The resonance wavelength in the structure will change in the first method when a physical sensor parameter is changed or when a biomolecule is connected to the biosensor. Such sensors are improved by reducing the full width at half maximum (FWHM).
In the second method, the resonant intensity of wavelength changes as the analyte is added to the sensing surfaces, and a physical sensor parameter is changed. Compared to the first procedure, these take fewer tools. This method's limitation to a narrow range of wavelengths is one of its disadvantages. The following has been done to analyze certain photonic crystal sensor parameters [68,69,70,71,72]:
2.1 Quality factor
One of the main parameters in a photonic crystal biosensor is the quality factor. This parameter can be obtained according to the following equation:
In the above relation \({\lambda }_{o}\) is the central resonance wavelength of the structure and \({\Delta \lambda }_{FWHM}\) is the full width at half maximum for the central spectrum.
2.2 Sensitivity
Changes in the connection of the analyte to a sensor will lead to changes in the signal transduction of the photonic crystal biosensor. The sensitivity of a biosensor can be obtained according to the following two equations.
In the above relation, \({\Delta \lambda }_{0}\) is the displacement of the transmission spectrum and m is the biomolecule weight.
In the above relation, Δn is the refractive index change.
2.3 Full-width at half-maximum (FWHM)
Due to this parameter, the wavelength distance between half of the output power can be obtained.
2.4 Free spectral range (FSR)
This range indicates the distance between the two resonance peaks at the output. This parameter plays an important role in the selectivity of a photonic crystal biosensor.
The next important parameter is DL, which is obtained according to Equation 14-16.
In the above relation, λ is equal to the resonance wavelength, S is equal to the sensitivity and Q is equal to the quality factor.
The next parameter is the competency factor or FOM, which is obtained according to Eqs. (14–16).
\(S\) is the sensitivity, \(Q\) is the quality factor, and \(\lambda\) is the same as the resonance wavelength in the equation above.
The design and simulation of PCRR (Photonic Crystal Ring Resonators) are facilitated by RSoft software. The simulations and calculations are conducted employing the finite-difference time-domain (FDTD) method. To utilize this method effectively, precise meshing and time calculations are imperative. The meshing size for the structure is set to \({\Delta }_{x}\)=\({\Delta }_{z}\)=25 nm, corresponding to the fixed size of the network, which is 600 nm. The time step equation is also taken into consideration [75].
Here, \({\Delta }_{t}\), \({\Delta }_{x}\), and \({\Delta }_{z}\) represents the time step, mesh size in the x-direction (\({B}_{x}\)), and mesh size in the z-direction, respectively. C represents the speed of light. With the number of time steps set to 5000 for each simulation, the time step required for calculations is determined as \({B}_{x}\)=0.016.
2.5 Photonic crystal biosensor
Electronics, optical communication, and biosensors are just a few fields in which photonic crystals are used. In photonic crystal-based biosensors, the refractive index of the photonic crystal structure is initially changed by the identification of the measured parameter. Accordingly, including the desired substance in the photonic crystal structure will change that region's refractive index, which will change the transmission spectrum [60,61,62,63, 79].
Based on the changes in the refractive index in the FDTD method [64, 80, 81], a photonic crystal biosensor is created to identify cholesterol, HIV, and sickle cell anemia. A blood sample is placed within the sensor's hole cavity, and a Gaussian light source with a 1.5–1.6 mm wavelength is injected into the waveguide.
A biosensor, based on dielectric rods in the air is introduced in Ref [65]. Between the waveguide input and output is an embedded oval-shaped nanoring in this structure. A dielectric rod is positioned between the input waveguide and the nano ring to raise the quality factor.
A one-dimensional photonic crystal defect-based biosensor is proposed in Ref [66]. The TMM approach has been used in this biosensor to investigate its performance. The concentration of blood keratin, defect layer thickness, and other information can be obtained using the parameters that were obtained.
A biosensor based on air holes in the silicon plate is introduced in Ref [67]. Defects in the shape of a capsule between two waveguides make up this biosensor. The center of the structure is where these breaches, which resemble capsules, are found. This sensor aims to determine the amount of concentration glucose in the human body.
The proposed structure consists of silicon dielectric rods in the air with a refractive index of 1. This structure is placed on a silicon dioxide plate with a refractive index of 1.46. In order to alleviate challenges related to absorption and material dispersion, it is preferable to use a silicon dielectric in an air configuration. In the RSoft software, this configuration is referred to as "pure silicon.
The intended structure comprises two waveguides, including an input and an output waveguide, with a nano-ring resonator sandwiched between them, which is a detecting region inside the nanoring. The lattice constant of the structure is 600 nm, while the cylinders' radius is 120 nm. We conducted over 300 manual simulations to fine-tune the structural parameters, carefully scrutinized the resulting values, conducted 100 sweeps to identify the optimal lattice constant and non-defect rods radius value based on bandgap analysis, ultimately determining them to be 600 nm and 120 nm, respectively.
This structure, along the X and Z axes, has \(23\times 17\) cylinders. The photonic crystal’s band structure with a direct bandgap along the \(\Gamma -\text{\rm M}-K-\Gamma\) of the first Brillouin zone is depicted in Fig. 2, and the desired sensor structure is shown in Fig. 3.
The band gap of this photonic crystal structure is in the range \(0.39<\text{a}/\uplambda <0.47\), which is within the range of the third telecommunication window. The gap has a wavelength distance of 1276 nm to 1538 nm. At the top of the band gap, the frequency levels in the \(\Gamma -\text{\rm M}\) path have a steeper slope and have less fluctuation in the \(\text{\rm M}-K\) path, which shows that the \(\text{\rm M}-K\) region has contributed more to the photonic gap. Also, at the edges of M and K, the frequency levels are perpendicular, indicating the formation of optical standing waves.
Two cylinders are placed in the input waveguide just before the light enters the resonator nano ring to increase the quality factor. The size of R0 in Fig. 3 is equal to R0 = radius × 0.5. First, a biomolecule of blood cholesterol with a refractive index of 1.5250 is connected to the sensing region, and the biosensor output is inspected. This output has been evaluated using 0.1 step changes in the blood cholesterol refractive index. In the first case, Nano cavity cylinders' radii are the same size as the structure's other cylinders. Table 2 displays the biosensor output after attaching the cholesterol molecule to the sensing region.
As shown in Table 2, we have a good resonance wavelength shift, high-quality factor, reasonably good sensitivity, high figure of merit, and low detection limit if the detecting cylinders around the sensing region are the same size as the other rods of the biosensor structure. The sensitivity, figure of merit, and resonance wavelength shift all rise as the cholesterol refractive index steps rise, but the detection limit and quality factor decrease. When cholesterol is attached to the detecting region, Fig. 4 shows each desired biosensor output separately.
As mentioned earlier, the refractive index of blood cholesterol is 1.5250 [64, 82], and in this structure, by connecting the biological molecule to the sensing region or changing the refractive index of the sensing cylinders, there are changes in the resonance wavelength shift and quality factor. Refractive index changes occur in steps of 0.1 from 1.025 to 1.5250. The biosensor's output is measured and its changes are noted for each change. Figure 5 depicts the outcomes of step changes to the structure.
As seen in the image above, a high resonant wavelength shift occurs when the biomolecule is connected to the biosensor sensing region, which improves and facilitates the identification of the target biomolecule.
The second stage's lattice constant is still 600 nm, but the sensing region's cylinders' radius has increased. The size of these cylinders is equal to R1 = R2 = R3 = R4 = R5 = R6 = radius × 1.5, which is equal to 180 nm. The desired structure is shown in Fig. 6.
All simulation processes were repeated after increasing the sensing region's cylinders, and the results are shown in Table 3 along with the output parameters.
Table 3 shows how increasing the radius of the cylinders surrounding the sensing region improves the structure's output results. The resonance wavelength shift and the detection limit have increased with an increase in the refractive index of blood cholesterol, whereas the figure of merit, sensitivity, and quality factor has decreased. When cholesterol is connected to the sensing region, Fig. 7 shows each output of the biosensor separately (stage 2).
As seen in the above figure, in addition to the resonance wavelength shift, the output power has also increased, which improves the ability of the detector section to identify the output wavelengths. Additionally, for a clearer comparison, Fig. 8 shows the resonance wavelength shift of the structure close to one another (stage 2).
Figure 8 shows that the resonance wavelength shift improves and increases when the refractive index approaches 1.5250. However, as the refractive index changes are gradually lessened, the output power increases.
Table 4 compares the proposed structure to prior works and evaluates the simulation results and other aspects.
Table 4 shows that the proposed structure has a higher quality factor and a higher figure of merit than other structures. Additionally, the detection limit has a low value. These parameters result from the change in cholesterol refractive index from 1.025 to 1.5250.
The possible implementation structure is depicted in Fig. 9. A laser is used as a broadband light source in this experimental setup. An objective lens couples the light into the polarization controller. The biosensor is fixed in a silicon dioxide plate. The transmitted light is then collected by a polarizer lens, and it will be obtained by a laptop after being detected by a detector.
3 Conclusion
A photonic crystal biosensor based on a nanoring resonator between the input and output waveguide is presented. In this biosensor, the dispersion and optical characteristics of light under changing refractive indices are calculated using the plane wave expansion (PWE) and finite difference in time domain (FDTD) methods. The refractive index in this biosensor gradually rose till it reached 1.525, and changes in the refractive index have been studied. Also, by increasing the radius of the cylinders around the sensing region, the biosensor output parameters have been improved. The obtained quality factor has a high value of 4988, and the figure of merit has also increased and has a value of 57.14 RIU−1. The detection limit in this structure is low and has a value of 0.001. The sensitivity obtained for this biosensor is equal to 32 (nm/RIU).
This paper has not reported experiments on live vertebrates or higher invertebrates.
Data availability
All relevant data are within the research paper.
References
Schoeneck M, Iggman D. The effects of foods on LDL cholesterol levels: a systematic review of the accumulated evidence from systematic reviews and meta-analyses of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2021;13:1325–38.
Wadhera RK, Steen DL, Khan I, Giugliano RP, Foody JM. A review of low-density lipoprotein cholesterol, treatment strategies, and its impact on cardiovascular disease morbidity and mortality. J Clin Lipidol. 2016;10:472–89.
Carson JAS, Lichtenstein AH, Anderson CAM, Appel LJ, Kris-Etherton PM, Meyer KA, Petersen K, Polonsky T, Van Horn L. Dietary cholesterol and cardiovascular risk: a science advisory from the american heart association. Ovid Technol (Wolters Kluwer Health). 2020;41:e39–53.
Ravnskov U, Diamond DM, Hama R, Hamazaki T, Hammarskjöld B, Hynes N, Kendrick M, Langsjoen PH, Malhotra A, Mascitelli L, McCully KS, Ogushi Y, Okuyama H, Rosch PJ, Schersten T, Sultan S, Sundberg R. Lack of an association or an inverse association between low-density-lipoprotein cholesterol and mortality in the elderly: a systematic review. BMJ Open. 2016;6: e010401.
Ueda P, Gulayin P, Danaei G. Long-term moderately elevated LDL-cholesterol and blood pressure and risk of coronary heart disease. PLOS ONE. 2018;13: e0200017.
Kopecky SL, Alias S, Klodas E, Jones PJH. Reduction in serum LDL cholesterol using a nutrient compendium in hyperlipidemic adults unable or unwilling to use statin therapy: a double-blind randomized crossover clinical trial. J Nutr. 2022;152:458–65.
Tang F, Wang J, Nicholas S, Qian D, Liu R. A cohort study on risk factors of high-density lipoprotein cholesterol hypolipidemia among urban Chinese adults. Lipids Health Dis. 2021. https://doi.org/10.1186/s12944-021-01449-1.
Davidson RM, Seneff S. The initial common pathway of inflammation, disease, and sudden death. Entropy. 2012;18:1399–442.
Sinning D, Landmesser U. Low-density lipoprotein-cholesterol lowering strategies for prevention of atherosclerotic cardiovascular disease: focus on siRNA treatment targeting PCSK9 (Inclisiran). Cur Cardiol Rep. 2020. https://doi.org/10.1007/s11886-020-01427-6.
Rosch PJ. Cholesterol does not cause coronary heart disease in contrast to stress. Scand Cardiovasc J. 2008;42:244–9.
Mortensen MB, Caínzos-Achirica M, Steffensen FH, Bøtker HE, Jensen JM, Sand NPR, Maeng M, Bruun JM, Blaha MJ, Sørensen HT, Pareek M, Nasir K, Nørgaard BL. Association of coronary Plaque with low-density lipoprotein cholesterol levels and rates of cardiovascular disease events among symptomatic adults. JAMA Netw Open. 2022;5: e2148139.
Kurstjens S, Gemen E, Walk S, Njo T, Krabbe J, Gijzen K, Elisen MG, Kusters R. Performance of commercially-available cholesterol self-tests. Ann Clin Biochem Int J Lab Med. 2021;58:000456322199239.
Getz G, Reardon C. Apoprotein E and reverse cholesterol transport. Int J Mol Sci. 2018;19:3479.
Chakraborty S, Doktorova M, Molugu TR, Heberle FA, Scott HL, Dzikovski B, Nagao M, Stingaciu L, Standaert RF, Barrera FN, Katsaras J, Khelashvili G, Brown MF, Ashkar R. How cholesterol stiffens unsaturated lipid membranes. Proc Natl Acad Sci. 2020;117:21896–905.
Law M, Wald N. Cholesterol, statins, and mortality. The Lancet. 2008;371:1161–2.
Zahedi A, Parandin F, Karkhanehchi MM, Habibi-Shams H, Rajamand S. Design and simulation of optical 4-channel demultiplexer using photonic crystals. J Opt Commun. 2017;40:17–20.
Abbasi M, Soroosh M, Namjoo E. Polarization-insensitive temperature sensor based on liquid filled photonic crystal fiber. Optik. 2018;168:342–7.
A. Askarian, F. Parandin, 2022 A novel proposal for all optical 1-bit comparator based on 2D linear photonic crystal. J Comput Electron. 2023;22:288–95.
Parandin F, Sheykhian A. Designing a circuit for high-speed optical logic half subtractor. Int J Circ, Syst Signal Process. 2022;16:887–91.
Parandin F, Sheykhian A. Design and simulation of a 2 × 1 all-optical multiplexer based on photonic crystals. Opt Laser Technol. 2022;151:108021.
M. Jalali, M. Soroosh, M. K. Moravvej Farshi and A. R. Nabavi, 2005. Transient and frequency analysis of PIN avalanche photodetector using circuit model. Proceedings of LFNM 2005. 7th International Conference on Laser and Fiber-Optical Networks Modeling, Yalta, Ukraine, pp. 294–296.
Salehnezhad Z, Soroosh M, Farmani A. Design and numerical simulation of a sensitive plasmonic-based nanosensor utilizing MoS2 monolayer and graphene. Diam Relat Mater. 2023;131: 109594.
Parandin F, Sheykhian A. Design of an all-optical half adder based on photonic crystal ring resonator. Opt Quant Electron. 2022. https://doi.org/10.1007/s11082-022-03810-9.
Sharma P, Sharan P. Design of photonic crystal-based ring resonator for detection of different blood constituents. Opt Commun. 2015;348:19–23.
Swain KP, Palai G. Estimation of human-hemoglobin using honeycomb structure: An application of photonic crystal. Optik. 2016;127:3333–6.
Robinson S, Dhanlaksmi N. Photonic crystal-based biosensor for the detection of glucose concentration in urine. Photonic Sens. 2017;7:11–9.
Bahabady AM, Olyaee S, Arman H. Optical biochemical sensor using photonic crystal nano-ring resonators for the detection of protein concentration. Cur Nanosci. 2017;13:421–5.
Tavousi A, Rakhshani MR, Mansouri-Birjandi MA. High sensitivity label-free refractometer-based biosensor applicable to glycated hemoglobin detection in human blood using all-circular photonic crystal ring resonators. Opt Commun. 2018;429:166–74.
Arunkumar R, Suaganya T, Robinson S. Design and analysis of 2D photonic crystal-based biosensor to detect different blood components. Photonic Sens. 2019;9:69–77.
Mohammeda NA, Hameda MM, Khalaf AAM, Alsayyari A, El-Rabaie S. High-sensitivity ultra-quality factor and remarkable compact blood components biomedical sensor based on nanocavity coupled photonic crystal. Result Phys. 2019;14:102478.
Biswas U, Kumar Rakshit J. Detection and analysis of hemoglobin concentration in blood with the help of photonic crystal based micro ring resonator structure. Opt Quant Electron. 2020;52:449.
Baraty F, Hamedi S. Design of a 2D photonic crystal biosensor using X-shape ring resonator based on Graphene Oxide (GO) for detection of blood components. J Electromagn Waves Appl. 2022. https://doi.org/10.1080/09205071.2022.2080004.
Parandin F, Heidari F, Aslinezhad M, Parandin MM, Roshani S, Roshani S. Design of 2D photonic crystal biosensor to detect blood components. Opt Quant Electron. 2022. https://doi.org/10.1007/s11082-022-03945-9.
Daher MG, Taya SA, Colak I, Vigneswaran D, Olaimat MM, Patel SK, Ramahi OM, Almawgani AHM. Design of a nano-sensor for cancer cell detection based on a ternary photonic crystal with high sensitivity and low detection limit. Chin J Phys. 2022;77:1168–81.
Khani S, Hayati M. Optical biosensors using plasmonic and photonic crystal band-gap structures for the detection of basal cell cancer. Sci Rep. 2022;12:5246.
Mittal S, Saharia A, Ismail Y, Petruccione F, Bourdine AV, Morozov OG, Demidov VV, Yin J, Singh G, Tiwari M. Spiral shaped photonic crystal fiber-based surface plasmon resonance biosensor for cancer cell detection. Photonics. 2023;10:230.
Hemanth Kumar BM, Srikanth PC, Vaibhav AM. A novel computation method for detection of malaria in RBC using photonic biosensor. Int J Inf Technol. 2021;13:2053–8.
Bhuvneshwer A, Bhargava S, Bhargava A. Biosensor application of one-dimensional photonic crystal for malaria diagnosis. Plasmonics. 2021;16:59–63.
Chaudhary VS, Kumar D, Kumar S. Gold-immobilized photonic crystal fiber-based SPR biosensor for detection of malaria disease in human body. IEEE Sens J. 2021;21:17800–7.
Mohammed NA, Hamed MM, Khalaf AAM, EL-Rabaie S. Malaria biosensors with ultra-sensitivity and quality factor based on cavity photonic crystal designs. Eur Phys J Plus. 2020. https://doi.org/10.1140/epjp/s13360-020-00940-5.
Shafkat A, Rashed ANZ, El-Hageen HM, Alatwi AM. Design and analysis of a single elliptical channel photonic crystal fiber sensor for potential malaria detection. J Sol-Gel Sci Technol. 2021;98:202–11.
Amiri IS, Paul BK, Ahmed K, Aly AH, Zakaria R, Yupapin P, Vigneswaran D. Tri-core photonic crystal fiber based refractive index dual sensor for salinity and temperature detection. Microw Opt Technol Lett. 2019;61:847–52.
Vigneswaran D, Ayyanar N, Sharma M, Sumathi M, Mani Rajan MS, Porsezian K. Salinity sensor using photonic crystal fiber. Sens Actuators A Phys. 2018;269:22–8.
Sayed H, Aly AH. Salinity optical sensor by using two-dimensional photonic crystals: computational study. Mater Sci Eng B. 2021;269: 115169.
Rabee ASH, Hameed MFO, Heikal AM, Obayya SSA. Highly sensitive photonic crystal fiber gas sensor. Optik. 2019;188:78–86.
Qin J, Zhu B, Du Y, Han Z. Terahertz detection of toxic gas using a photonic crystal fiber. Opt Fiber Technol. 2019;52: 101990.
Asaduzzaman S, Ahmed K, Bhuiyan T, Farah T. Hybrid photonic crystal fiber in chemical sensing. SpringerPlus. 2016. https://doi.org/10.1186/s40064-016-2415-y.
Clerk-Maxwell J. A dynamical theory of the electromagnetic field. Philos Trans Roy Soc London. 1865;155:459–512.
Parandin F, Karkhanehchi MM. Low size all optical XOR and NOT logic gates based on two-dimensional photonic crystals. Majlesi J Electr Eng. 2019;13:1–5.
Parandin F, Kamarian R, Jomour M. Designing an optical 1-bit comparator based on two-dimensional photonic crystals. Appl Opt. 2021;60:2275–80.
Parandin F, Olyaee S, Kamarian R, Jomour M. Design and simulation of linear all-optical comparator based on square-lattice photonic crystals. Photonics. 2022;9:459.
Maleki MJ, Mir A, Soroosh M. Designing an ultra-fast all-optical full-adder based on nonlinear photonic crystal cavities. Opt Quant Electron. 2020;52:196.
Karkhanehchi MM, Parandin F, Zahedi A. Design of an all-optical half-adder based on 2D photonic crystals. Photon Netw Commun. 2017;33:159–65.
Bostan CG, Ridder RMD. Design of photonic crystal slab structures with absolute gaps in guided modes. J Opt Adv Mat. 2002;4:921–8.
Joannopoulos JD, Johnson SG, Winn JN, Meade RD. Molding the flow of light. Princeton, NJ: Princeton Univ. Press; 2008.
Miyan H, Agrahari R, Gowre SK, Mahto M, Jain PK. Computational study of a compact and high sensitive photonic crystal for cancer cells detection. IEEE Sens J. 2022;22:3298–305.
Olyaeea S, Naraghi A. Design and optimization of index-guiding photonic crystal fiber gas sensor. Photon Sens. 2013;3:131–6.
Olyaee S, Seifouri M, Karami R. Designing a high sensitivity hexagonal nano-cavity photonic crystal resonator for the purpose of seawater salinity sensing. Opt Quant Electron. 2019;97:2–9.
Parandin F, Moayed M, Heidari F. All-optical diode for terahertz optical power rectification based on two-dimensional photonic crystals. Microelectron J. 2023;136: 105779.
Yang X, Lu Y, Liu B, Yao J. Fiber ring laser temperature sensor based on liquid-filled Photonic crystal fiber. IEEE Sens J. 2017;17:6948–52.
Inan H, Poyraz M, Inci F, Lipson MA, Baday M, Cunningham BT, Demirci U. Photonic crystals: emerging biosensors and their promise for point-of-care applications. Chem Soc Rev. 2017;46:366–88.
Zhang Q, Zhong L, Tang P, Yuan Y, Liu S, Tian J, Lu X. Quantitative refractive index distribution of single cell by combining phase-shifting interferometry and AFM imaging. Sci Rep. 2017;7:1–10.
Yablonovitch E. Photonic band-gap structures. JOSA B. 1993;10:283–95.
Hemanth Kumar BM, Vaibhav AM, Srikanth PC. Si/SiO2 based nano-cavity biosensor for detection of anemia, HIV and cholesterol using refractive index of blood sample. Indian J Sci Technol. 2022;15:899–907.
Arunkumar R, Suaganya T, Robinson S. Design and analysis of 2D photonic crystal-based biosensor to detect different blood components. Photonic Sens. 2019;9:69–77.
Aly AH, Mohamed D, Mohaseb MA, Abd El-Gawaad NS, Trabelsi Y. Biophotonic sensor for the detection of creatinine concentration in blood serum based on 1D photonic crystal. RSC Adv. 2020;10:31765–72.
Maache M, Fazea Y, Hassan IB, Alkahtani AA, Ud Din I. High-sensitivity capsule-shaped sensor based on 2D photonic crystals. Symmetry. 2020;12:1480.
Parandin F, Heidari F, Rahimi Z, Olyaee S. Two-dimensional photonic crystal biosensors: a review. Opt Laser Technol. 2021;144:107397.
Sani MH, Khosroabadi S. A novel design and analysis of highsensitivity biosensor based on nano-cavity for detection of blood component, diabetes, cancer and glucose concentration. IEEE Sens J. 2020;20:7161–8.
Parandin F, Rahimi Z, Rezaeenia M. Design of an ultra-compact photonic crystal based all optical XOR and NOT logic gates. J Opt Commun. 2022. https://doi.org/10.1515/joc-2022-0137.
Parandin F, Heidari F. Design and simulation of a biosensor based on 2D photonic crystal nano-ring resonator. Nashriyyah -I Muhandisi -I Barq Va Muhandisi -I Kampyutar -I Iran, Amuhandisi -I Barq. 2020;18:129–32.
Parandin F, Olyaee S, Heidari F, Soroosh M, Farmani A, Saghaei H, Karimzadeh R, Maleki MJ, Askarian A, Rahimi Z, Ehyaee A. Recent advances in all-optical half-subtractor and full-subtractor based on photonic crystal platforms. J Opt Commun. 2024. https://doi.org/10.1515/joc-2023-0314.
Kumar Chhipa M, Madhav BTP, Suthar B, Janyani V. Ultra-compact with improved data rate optical encoder based on 2D linear photonic crystal ring resonator. Photon Netw Commun. 2022;44:30–40.
Chhipa MK, Madhav BT, Savarimuthu R, Vijay J, Suthar B. Realization of all-optical logic gates using a single design of 2D photonic band gap structure by square ring resonator. Opt Eng. 2021;60:075104.
BV Rajarajan, M Murugan, R Savarimuthu, R Nakkeeran, 2017. Opt. Quantum Electron. 49
Chhipa MK, Madhav BTP, Suthar B. An all-optical ultracompact microring-resonator-based optical switch. J Comput Electron. 2021;20:419–25.
Parandin F, Rahimi Z, Moloudi A, Heidari F, Parandin MM. A comprehensive review of blood component detection utilizing one-dimensional, two-dimensional, and photonic crystal fiber biosensors. Result Opt. 2024. https://doi.org/10.1016/j.rio.2024.100671.
Chhipa MK, Madhav BTP, Suthar B. Design and analysis of optical filter for optical communication networks using photonics technology. AIP Conf Proc. 2020;2220:050020.
Radhouene M, Najjar M, Chhipa MK, Robinson S, Suthar B. “Design and analysis a thermo-optic switch based on photonic crystal ring resonator. Optik. 2018;172:924–9.
Radhouene M, Chhipa MK, Najjar M, Robinson S, Suthar B. Novel design of ring resonator-based temperature sensor using photonics technology. Photonic Sens. 2017;7:311–6.
Chhipa MK, Radhouene M, Robinson S, Suthar B. Improved dropping efficiency in two-dimensional photonic crystal-based channel drop filter for coarse wavelength division multiplexing application. Opt Eng. 2017;56: 015107.
Kumar A, Verma P, Verma P. Photonic crystal fiber based refractive index sensor for cholesterol sensing in far infrared region. Adv Data Comput Commun Secur. 2022. https://doi.org/10.1007/978-981-16-8403-6_49.
Funding
The authors have no relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Contributions
F. Heidari, F. Parandin, Z. Rahimi and M.M. Parandin simulated the structure and prepared the paper. A. Boochani edited the article scientifically.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All authors were cooperative and involved in research activities of this article.
Consent for publication
All authors agreed to publish this article.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Heidari, F., Parandin, F., Boochani, A. et al. Design of a two-dimensional photonic crystal biosensor to identify blood cholesterol in humans. Discov Electron 1, 4 (2024). https://doi.org/10.1007/s44291-024-00003-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44291-024-00003-4