Abstract
Recently, Spodoptera litura (Fab.) has emerged as a significant threat to the environment. Because of the pest's ability to spread, pesticides are often applied to agricultural crops. By combining certain plant extracts with other compounds, both a reduction in insect infestation and an increase in production could be achieved. The antifeedant, larvicidal and histological effects of Solanum torvum (Sw.) leaf extracts were investigated against S. litura. The study found that the ethyl acetate leaf extract showed a significant antifeedant effect against S. litura of (86.16%) at 5% concentration. Ethyl acetate extract showed larvicidal activity against S. litura of 88.21% and the LC50 value was 2.05%. Exposure of larvae to ethyl acetate leaf extract resulted in significant histological damage, particularly affecting epithelial, goblet and digestive cells. The results suggest that the inclusion of these plant extracts in integrated pest management approaches can promote sustainable and environmentally friendly pest control methods in agriculture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The devastating effects of Spodoptera litura (Fab.), commonly known as tobacco worm, on agricultural and vegetable crops have been observed in numerous countries such as India, China and Japan. This voracious pest poses a significant threat to crop yields and food security and requires extensive research efforts to understand its biology, behaviour and management strategies. The researchers found that 181 plant species from 39 different families were affected [1]. Various categories of chemical insecticides have emerged as the predominant approach to pest management and control. The indiscriminate use of synthetic compounds can lead to several dangerous consequences, including environmental pollution, insect species toxicity and genetic resistance, among others.
For many years, the use of various pesticide sprays has been the main method of controlling S. litura. Numerous field populations of S. litura have become highly resistant to a variety of insecticides, including pyrethroids, carbamate, organophosphates, and some newer chemical insecticides, including indoxacarb, abamectin, emamectin benzoate, and chlorantraniliprole [2]. Worldwide, the fight against this pest is becoming increasingly difficult. In order to develop efficient resistance management techniques, it is crucial that we understand the mode of action and resistance mechanisms of insecticides. Furthermore, elucidating the molecular mechanism behind pesticide resistance will open new avenues for the development of cutting-edge pest control techniques.
Plant extracts and secondary metabolites have emerged as promising alternatives to synthetic pesticides, offering potential solutions for sustainable pest management strategies. In recent years, there has been growing interest in the potential of essential oils as a viable alternative to synthetic pesticides [3]. The utilization of plant pesticides offers several notable benefits, primarily attributed to their swift degradation and minimal persistence and bioaccumulation within the ecosystem [4].
Solanum torvum (Sw.) from the Solanaceae family was used for this research. They are widespread in India, Malaysia, Pakistan, tropical America, etc., [5]. Furthermore, an extensive literature search revealed no scientific publications on pest management or control, although locals have long used this plant as food and medicine to combat intestinal parasite larvae and dental problems [6]. In addition, it is used to treat contact and repellent activity against Callosobruchus maculatus (Fab.) [7] and larvicidal and adulticidal activity against Aedes aegypti (L.) [8, 9]. In the present work S. torvum leaf extracts, we report the antifeedant, larvicidal and histological examination against S. litura (Fig. 1).
2 Materials and methods
2.1 Plant material
In 2023, leaves from a healthy Solanum torvum were collected from Kovilvenni, near the Thanjavur district. Reverend Dr. S. John Brito, Director of the Center for Molecular Systems at St. Joseph's College (Autonomous) in Tiruchirappalli, Tamil Nadu, India, confirmed its classification and validity. The specimens are stored at the Robinot Herbarium, with the voucher number ST004. To prepare the leaves for experimental investigation, they were dried, stored in an airtight container, and finally processed.
2.2 Preparation of plant extract
The S. torvum leaves were collected, allowed to dry in the shade at room temperature, and then ground with a mixer grinder. For 48 h, 3 L (1:3 w/v) of hexane, ethyl acetate, and methanol were used to extract the powder (1 kg). The extract was filtered using Whatman No. 1 filter paper and a Buchner funnel. The filtrate was dried using a rotary evaporator (Model RE 801, Yamato, Japan) at 40 °C and reduced pressure. According to Murugesan et al. [9], the crude extracts were kept cold until needed.
2.3 Mass culture of Spodoptera litura
The S. litura culture was maintained in the Research Department of Zoology and Biotechnology, A.V.V.M. Sri Pushpam College (Autonomous), Poondi, Thanjavur. Spodoptera litura larvae were reared in plastic containers lined with paper towels that could absorb moisture. They received a 10% honey solution specifically tailored to their nutritional needs. A fresh meal was served every two to three days to maximize nutrition and reduce infection. To maintain rearing conditions, a controlled environment chamber with the following settings was used: 25 ± 1 °C temperature, 60—70% relative humidity, and a 14:10 light–dark cycle. To reduce stress-related responses, larvae were given 48—72 h to acclimate to the laboratory environment before beginning the experiments [10].
2.4 Antifeedant activity
The antifeedant effect of the S. torvum leaves crude extracts was examined using the leaf disc no-choice method [11]. Different concentrations of crude extracts were prepared by dissolving them in acetone and testing them against S. litura. Fresh castor leaf discs with a diameter of 4 cm were punched out using a cork drill. They were then individually immersed in crude extract concentrations of 0.625, 1.25, 2.5, and 5%. Since acetone was used to dissolve the crude extracts, the leaf discs dipped in the substance were a negative control. To prevent the leaf discs from drying out too early, wet filter paper was placed in each 1.5 cm × 9 cm plastic petri dish and a single third instar larva was placed in each petri dish. The progressive leaf consumption of the treated and control larvae was recorded over 24 h using a leaf area meter (Delta-T Devices, S. No: 15736 F 96, UK). The number of leaves eaten by the treatment larvae was adjusted based on the negative control. For each treatment, five replicates each containing 10 larvae were kept.
C and T denote the relative amounts of leaf consumed by the larvae in the control and treated discs.
2.5 Larvicidal activity
Different concentrations of crude extracts were applied using the leaf dipping method. The larvae were exposed to the treated leaves. The larvae were kept alive on fresh castor bean leaves that had not been treated for a whole day. Fresh castor leaves were delivered every 24 h. Death of the larvae was noted 96 h after treatment. For each treatment, five replicates containing ten larvae each were maintained. The mortality percentage was calculated using Abbot method [12]. The experiment was carried out with a light photoperiod 14:10, a laboratory temperature of 27 ± 2 ºC and a relative humidity of 75 ± 5%.
2.6 Histological analysis in the midgut of Spodoptera litura
The digestive system of S. litura was used for the histological examination. The abdominal part of the larval parts was fixed in 10% neutral buffered formalin. The tissue samples were dehydrated and cleaned using a tissue processor. The samples were then fixed in paraffin blocks using a rotary microtome and an embedding station, 4 μm thick sections were cut (Leica RM2255 microtome, Buffalo Grove, IL, USA), and stained with hematoxylin and eosin stains. Finally, the stained sections of the tissue sample of S. litura larvae were examined using light microscopy [13].
2.7 Statistical analysis
The mortality data were subjected to one-way analysis of variance (ANOVA) with Tukey's multiple range tests to find the effective treatment at p < 0.05 for further binary mixture studies and to determine the significant difference between treatments. Probit analysis calculated the lethal concentration for 50% mortality (LC50) for a 96-h exposure period. Probit analysis was performed using SPSS statistical software (Version 16.0).
3 Results
3.1 Antifeedant and larvicidal activity
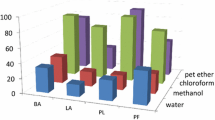
The present study deals with the antifeedant and larvicidal activities of hexane, ethyl acetate, and methanol extracts of S. torvum against S. litura. The antifeedant activity of S. torvum crude extract was tested against S. litura, and the results were presented in Table 1. The extract hexane and methanol showed moderate antifeedant activity (51.33%), (77.16%) and ethyl acetate extract showed significant activity (86.16%) at 5% concentration. Ethyl acetate exhibited larvicidal activity of 88.21% with an LC50 value of 5%. The larvae, after treatment with ethyl acetate extract, showed mortality in larval, and adult stages (Tables 2 and 3).
3.2 Histological analysis in the midgut of Spodoptera litura
Histological analysis was observed 72 h after exposure in the midgut of 3rd instar S. litura larvae treated with a 5% concentration of S. torvum leaf extract. A significant effect was observed when the larvae were treated with 5% of ethyl acetate extract. The striated border and peritrophic membrane of the epithelial cells disrupted. The maximum damage was observed in larvae exposed to ethyl acetate leaf extract. Epithelium and significant vacuolization in the goblet and digestive cells of the midgut (Fig. 2).
Histological examination of 3rd instar larvae of Spodopter litura (Fab.) (Solanum torvum (Sw.): A control; B hexane extract; C ethyl acetate extract; D methanol extract treatments). Muscular Layer (ML), Membrane Peritrophic (MP), Epithelial Cell (EC), Basal Membrane (MB) and disintegration (D), CC- Columnar cells, RC- Regenerative cells, G- Goblet cell, Mv- Microvilli, and Pm-Peritropic membrane
4 Discussion
Many plants are reported to have antifeedant, growth inhibition, and larvicidal effects against S. litura, such as Eg., Zingiber officinale (R.), Pedalium murex (L.), Vitex negundo (L.), Citrus sinensis (L.) [14], Melia dubia (Cav.) [15], Ocimum basilicum (L.) [16]. Koul et al. [17] found that the extract of Aglaia elaeagnoidea (B.) had maximum antifeedant and larvicidal activity against Helicoverpa armigera (Hub.) and Pieris rapae (L.). Solanum torvum plant was studied with Culex quinquefasciatus (S.) larvae achieved maximum larvicidal activity [18]. Pavunraj et al. [19] stated that ethyl acetate leaf extract of Pergularia daemia (For.) has good antifeedant activity against S. litura. Salinas-Sanchez et al. [20] reported that Tagetes erecta (L.) hexane extract at 48%, acetone extract at 60% and ethanol extract at 500 ppm had a concentration of 72% compared to S. litura larvae. The results of Bhatt et al. [21] were closely related to our result. It was found that Dendrophthoe falcata (L.f.) leaves had an activity of 98.58%, Azadirachta indica (A.) fruit extract of 85.72% and Correa reflexa (Lab.) extract of 98.58%.
The findings of the present study reveal the antifeedant and larvicidal activities of hexane, ethyl acetate, and methanol extracts of S. torvum against S. litura larvae. The results demonstrate that the ethyl acetate extract exhibited significant antifeedant activity, reducing feeding by 86.16% at a 5% concentration, whereas the hexane and methanol extracts showed moderate antifeedant effects. This highlights the potential of ethyl acetate extract as a potent deterrent against S. litura feeding behaviour, suggesting its efficacy as a natural biopesticide.
The study's most important discovery was that the antifeedant treatment with the highest effectiveness also had the highest larvicidal activity. Many plants have been used for their larvicidal activity against S. litura, including Clerodendrum calamitosum (L.), Clerodendrum viscosum (L.), Clerodendrum multiflorum (L.), Clerodendrum philippinum (L.), Clerodendrum serratum (L.), Clerodendrum paniculatum (L.) and Clerodendrum splendens (L.) [22]. These results support the validation of Jbilou et al. [23] that acetone and ethanol extracts of Anticarsia gemmatalis (Hub.) contained potential insecticidal agents to control Agonoscelis pubescens (Thu.). Acorus calamus (L.) ethyl acetate leaf extract at 5% concentration 40.24% and Annona squamosa at 40% larval mortality was achieved against S. litura [24].
Moreover, the larvicidal activity of the ethyl acetate extract was notable, with a mortality rate of 88.21% and an LC50 value of 5%, indicating its effectiveness in controlling S. litura larvae. The observed mortality in both larval and adult stages further underscore the broad-spectrum efficacy of the ethyl acetate extract, making it a promising candidate for integrated pest management strategies. The leaf extract of Marrubium vulgare (L.) showed 42.2% larval mortality at a concentration of 5.0%. The larvae eventually stopped feeding, resulting in a developmental arrest at various larval instar stages [16]. The herbal insecticides and antifeedants can be essential components of an integrated pest management program.
Histological analysis of the midgut of S. litura larvae treated with the ethyl acetate extract revealed significant structural alterations, particularly in the epithelial cells. Disruption of the striated border and peritrophic membrane, along with vacuolization in the goblet and digestive cells, indicates the cytotoxic effects of the extract on the midgut tissues. These histopathological changes likely contribute to the observed larvicidal activity by compromising the integrity and functionality of the digestive system, ultimately leading to larval mortality.
The peritrophic membrane of S. litura larvae remained intact in the midgut region, and the untreated larvae showed signs of midgut architecture. Regenerative cells adhere to the surface of the basement membrane. Well-developed goblet cells and longitudinal muscle layers follow the epithelial cells, which have an elongated nucleus and decondensed chromatin. The epithelial cells have a densely packed, striated brush border and form columnar epithelial cells (Fig. 1). This condition shows that the proper functioning of the midgut cells enables the metabolic activity of the insect larvae [13]. The effect was found to be dose-dependent. The current results were consistent with those reported by Fiaz et al. [25] and were found in the midgut digestive cells of Anticarsia gemmatalis (Hub.) and S. litura larvae.
In insects, digestion and food absorption occur in the midgut area, which is of endodermal origin [26]. The midgut is a crucial part of the alimentary canal and takes up much space in the hemocoel. In addition, it plays a vital role in many physiological controls such as blood flow, immunological response, and metabolism [27]. In addition, azadirachtin has been found to cause specific histological changes in the body tissues of insects [28]. When plant pesticides were applied to Schistocerca gregaria (For.) and Locusta migratoria (L.), Cottee [29] noted changes such as cell necrosis, vacuolization of the cytoplasm, reduction in nuclear size, and cell regeneration. Therefore, interfering with these processes can serve as a target or strategy for subsequent pest control measures.
5 Conclusion
In conclusion, our research highlights the diverse potential of S. torvum leaf extracts and their transformative effect on managing S. litura larvae. These findings not only expand our understanding of natural pest control but also provide opportunities for developing innovative, environmentally friendly solutions in the agricultural sector. We look forward to further research and application of these findings in pursuing sustainable pest control.
Data availability
The author declares that, the data that used and/or analyzed during this study will be available from corresponding author on reasonable request.
References
Yooboon T, Pengsook A, Ratwatthananon A, Pluempanupat W, Bullangpoti V. A plant-based extract mixture for controlling Spodoptera litura (Lepidoptera: Noctuidae). Che Bio Techn Agri. 2019;6(1):1–10. https://doi.org/10.1186/s40538-019-0143-6.
Shi L, Shi Y, Zhang Y, Liao Xiaolan. A systemic study of indoxacarb resistance in Spodoptera litura revealed complex expression profiles and regulatory mechanism. Sci Rep. 2019;9:14997. https://doi.org/10.1038/s41598-019-51234-5.
Vendan SE, Manivannan S, Sunny AM, Murugesan R. Phytochemical residue profiles in rice grains fumigated with essential oils for the control of rice weevil. PLoS ONE. 2017. https://doi.org/10.1371/journal.pone.0186020.
Senthil NS. Physiological and biochemical effect of neem and other Meliaceae plants secondary metabolites against Lepidopteran insects. Front Physiol. 2013;4:359.
ZubaidaYousafa B, Wanga Ying, Baydounc Elias. Phytochemistry and pharmacological studies on Solanum torvum Swartz. J App Pharm Sci. 2013;3(04):152–60.
Murugesan R, Vasuki K, Kaleeswaran B, Santhanam P, Ravikumar S, Alwahibi MS. Insecticidal and repellent activities of Solanum torvum (Sw.) leaf extract against stored grain pest, Callosobruchus maculatus (F.) (Coleoptera: Bruchidae). J K Sa Uni Sci. 2021. https://doi.org/10.1016/j.jksus.2021.101390.
Murugesan R, Vasuki K, Kaleeswaran B, Ramadevi S, Thirumalai Vasan P. Environmentally benign solanum torvum (Sw.) (Solanaceae) leaf extract in Ecofreindly management of human disease vector Aedes aegypti (Linn.). J Biol Cont. 2022. https://doi.org/10.18311/jbc/2021/28195.
Murugesan R, Vasuki K, Kaleeswaran B. A green alternative: evaluation of solanum torvum (Sw.) leaf extract for control of Aedes aegypti (L.) and its molecular docking potential. Intell Pharm. 2023. https://doi.org/10.1016/j.ipha.2023.11.012.
Murugesan R, Vasuki K, Kaleeswaran B, Ramadevi S. A study on developmental toxicity and behavioural safety using ethanolic extract of Pedalium murux L. on zebrafish embryos. Adv Chemicobio Res. 2023;2(1):54–61. https://doi.org/10.37256/acbr.2120231958.
Liu NY, Yang K, Liu Y, Xu W, Anderson SL. Dong, two general-odorant binding proteins in Spodoptera litura are differentially tuned to sex pheromones and plant odorants. Comp Biochem Physiol. 2015;180:23–31.
Isman MB, Koul O, Lucyzynski A, Kaminski J. Insecticidal and antifeedant bioactivities of neem oils and their relationship to Azadirachtin content. J Agric Food Chem. 1997;38:1407–11.
Abbott WS. The value of the dry substitutes for liquid lime. J Eco Ento. 1925;18:265–7.
Suryani AI, Hariani N, Majid AF, Amalia DN. 2020 Histological changes in the midgut of Spodoptera litura larvae exposured by the extract of Mirabilis jalapa leaves. IOP Conference Series: Earth Envi Sci 484: (1).
Sahayaraj K. Antifeedant effect of some plant extracts on Asian armyworm, Spodoptera litura (Fabricius). Curr Sci. 1998;74(6):523–5.
Koul O, Jain M, Sharma V. Growth inhibitory and antifeedant activity of extracts from Meliadubia to Spodoptera litura and Helicoverpa armigera larvae. Ind J Exp Biol. 2000;38:63–8.
Pavela R. Insecticidal activity of certain medicinal plants. Fitoterapia. 2004;75:745–9.
Koul O, Singh G, Singh R, Singh J, Daniewski W, Berlozecki S. Bio efficacy and mode of action of some limonoids of salann in group from Azadirachta indica a. juss and their role in a multi component system against lepidopteran larvae. J Biosci. 2004;29:409–16.
Rahuman AA, Gopalakrishnan G, Venkatesan P, Geetha K. Isolation and identification of mosquito larvicidal compound from Abutilon indicum (Linn.) Sweet. Parasitol Res. 2008B;102:981–8.
Pavunraj M, Chellaiah M, Ignacimuthu S, Janarthanan S, Duraipandiyan V, Raja N, Vimalraj S. Antifeedant activity of a novel 6-(4, 7-hydroxy-heptyl quinine from the leaves of the milkweed Pergularia daemia on the cotton bollworm Helicoverpa armigera (Hub.) and the tobacco armyworm Spodoptera litura (Fab.). Phytoparasitica. 2011;39:145–50.
Salinas Sanchez DO, Aldana Llanos L, Valdes Estrada ME, Gutierrez Ochoa M, Valladares Cisneros G, Rodríguez FE. Insecticidal activity of Tagetes erecta extracts on spodoptera frugiperda (Lepidoptera: Noctuidae). Flor Entomo. 2012;95(2):428–32. https://doi.org/10.1653/024.095.0225.
Bhatt P, Thodsare N, Srivastava RP. Toxicity of some bioactive medicinal plant extracts to Asian army worm Spodoptera litura. J App Nat Sci. 2014;6(1):139–43. https://doi.org/10.31018/jans.v6i1.390.
Jadhav GS, Devarshi AA, Yankanchi SR. Efficacy of certain Clerodendrum leaf crude extracts against cutworm, Spodoptera litura Fab and cotton bollworm, Helicoverpa armigera Hub. J Entomo Zoo Stu. 2016;4(4):466–72.
Jbilou R, Ennabili A, Sayah F. Insecticidal activity of four medicinal plant extracts against Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). African J Biotechnol. 2006;5:936–40.
Raja N, Elumalai K, Jayakumar M, Jeyasankar A, Muthu C, Ignacimuthu S. Biological activity of different plant extracts against armyworm, Spodoptera litura (Fab) (Lepidoptera: Noctuidae). J Entomol Res. 2003;27:281–92.
Fiaz M, Martínez LC, da Silva CM, Cossolin JFS, Plata Rueda A, Goncalves WG, Serrao JE. Squamocininduce histological and ultrastructural changes in the midgut cells of Anticarsiagemmatalis (Lepidoptera: Noctuidae). Ecotoxicol Environ Saf. 2018;156:1–8.
Caccia S, Casartelli M, Tettamanti G. The amazing complexity of insect midgut cells: types, peculiarities, and functions. Cell Tissue Res. 2019;377(3):505–25.
Takeda M. Structures and functions of insect mid-gut: the regulatory mechanisms by peptides, proteins and related compounds, hemolymph proteins and functional peptides. R Adv Ins Arth. 2012;1:94–110.
Rembold H. The azadirachtins highly active insect growth inhibitors. recent advances in Medical. Aro Spice Crops. 1991;1:3–37.
Cottee PK. 1984. A physiological investigation into the role of secondary plant compounds as feeding derrents. Ph.D. Thesis University of Aberdeen UK.
Acknowledgements
Each author would like to thank their institution for the opportunity to develop this manuscript. The authors would like to thank the management of A. V. V. M. Sri Pushpam College (Autonomous), Thanjavur, for taking the time to write this research article.
Funding
There is no fund support for doing this research.
Author information
Authors and Affiliations
Contributions
RM Writing—review & editing, Writing—original draft, Software, Methodology, Funding acquisition, Formal analysis, Conceptualization, Data curation, Investigation. KV Conceptualization, Data curation. BK Investigation, Formal analysis, Methodology, Supervision, Validation.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rengarajan, M., Kumar, V. & Balasubramanian, k. Bio-efficacy of Solanum torvum (Sw.) against agricultural pest Spodoptera litura (Fab.) (Lepidoptera: Noctuidae). Discov Agric 2, 40 (2024). https://doi.org/10.1007/s44279-024-00035-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44279-024-00035-0