Abstract
The effects of crude extracts and an isolated compound from the leaves of milkweed, Pergularia daemia (Forssk) Choiv., on the antifeedant activity against two important lepidopteran pests, Helicoverpa armigera (Hub.) and Spodoptera litura (F.), were studied. Maximum antifeedant activity was recorded in ethyl acetate crude extract against H. armigera (70.3%) and S. litura (71.82%) at 1% concentration. Ethyl acetate crude extract was further subjected to column chromatography, which was performed using hexane as initial solvent and then by increasing the polar strength using ethyl acetate. Fractions collected at hexane and ethyl acetate (80:20) yielded 6-(4,7-hydroxy-heptyl) quinone, a novel compound which showed significant antifeedant activity against H. armigera (80.22% at 2000 ppm) and S. litura (68.31% at 2000 ppm).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemical pesticides play a significant role in increasing agricultural production by controlling the insect pests. However, the chronic effects of chemical pesticides on living organisms and the environment prompt us to restrict the use of many pesticides (Isman et al. 1990; Katyal and Satake 1996). The threats posed by chemical pesticides demand an urgent search for an environmentally safer alternative method of crop protection. The injudicious use of synthetic pesticides can lead to secondary outbreaks of pests that are normally under natural control, resulting in their rapid proliferation. There have also been cases of pests becoming tolerant to insecticides, resulting in the use of double and triple application rates (Stoll 1988). In addition, problems such as health hazards, undesirable side effects and environmental pollution are caused by the continuous use of synthetic chemical pesticides. There is renewed interest in the application of botanical pesticides for crop protection (Nas 2004). Scientists are now experimenting and working to protect insect infestation by indigenous plant materials. The use of locally available plants in the control of pests is an ancient technology in many parts of the world (Roy et al. 2005). Most of these botanical pesticides are non-selective poisons that target a broad range of pests. Especially, the secondary metabolites of several plants are found to be effective alternatives to synthetic pesticides (Leatemia and Isman 2004). The mode of action of these secondary metabolites has been studied in detail by many investigators. The action of the plant-derived compounds on pest insects is exerted through many ways such as antifeedant (Raja et al. 2005), larvicidal (Kabaru and Gichia 2001), ovicidal and oviposition deterrent activities (Pavunraj et al. 2006), repellent (Schmutterer 1995) and others. Some botanicals have an effect on juvenile hormone and ecdysone actions, but not on the hormones themselves (Williams et al. 1986). They also have substances that disrupt insect growth by antagonizing juvenile hormone action (Bowers et al. 1976).
The cotton bollworm H. armigera (Hübner) (Lepidoptera: Noctuidae) is a polyphagous pest of worldwide occurrence inflicting crop damage in India to the sum of one billion dollars annually (Subramaniyan and Mohankumar 2006). In India, this insect occurs as a major pest in many economically important crops including cotton, pigeonpea, chickpea, tomato, okra, and blackgram. The tobacco armyworm S. litura (Fabricius) (Lepidoptera: Noctuidae) was once recognized as a major pest of tobacco alone and now it has become a serious pest also of tomato (Alam 1969), cotton (Moussa et al. 1960), castor (Macleod et al. 1990) and bitterguard (Yasui et al. 1998). Recently, an outbreak of this pest was noticed in Kancheepuram District of Tamil Nadu, India, on brinjal, which is not a host plant of this pest.
The milkweed Pergularia daemia (Forssk.) Choiv. (Gentianales: Asclepiadaceae) is an important medicinal plant. Leaf juice of P. daemia is applied to treat rheumatic swelling and also used in the preparation of purgative medicinal oil given for rheumatism, amenorrhoea and dysmenorrhoea; the root bark is also used as a purgative in rheumatic cases (Wealth of India 1966). The root is used to treat mental disorders, anemia, leprosy, piles, and uterine and menstrual disorders (Yoganarasimhan 2000). The entire plant is used for pulmonary afflictions, asthma, biliousness, cough, piles, leprosy and syphilis, and leaves are used in infantile diarrhea, and as an expectorant, uterine tonic and emetic (Mohammed et al. 2004). The leaf aqueous extract of P. daemia showed some promising larvicidal activity against H. armigera (Ramya and Jayakumararaj 2009). Sakthivadivel and Danial (2008) reported that the petroleum crude extracts of P. daemia leaf exhibited a toxic effect on 4th instar larvae of some vector mosquitoes like Culex quinquefasciatus, Anopheles stephensi and Aedes aegypti. The present study was carried out with the objective of screening the extracts and a compound from P. daemia for their antifeedant activity against H. armigera and S. litura.
Materials and methods
Plant material
The leaves of P. daemia were collected during October 2007 from Salem district of Tamil Nadu, India. A voucher specimen (LC/ERI/Herb.205) was deposited in the herbarium of the Entomology Research Institute, Loyola College, Chennai, India.
Extraction of crude extracts
The leaves were dried in the shade at room temperature and ground in a hand mill. Two kilograms of plant material was powdered using an electric blender and 500 g leaf powder was soaked in 1.5 l of n-hexane in an aspirator bottle for 72 h with occasional shaking. Then the content of the aspirator bottle was filtered using Whatman No.1 filter paper and the filtrate was concentrated using a rotary vacuum evaporator at 40°C until further use. The remaining residue was sequentially extracted with chloroform and ethyl acetate.
Isolation of active compounds
Ten grams of crude ethyl acetate extract of P. daemia leaves were subjected to silica gel (60–120 and 230–400 mesh, Acme Synthetic Chemicals, Maharashtra, India) chromatography and eluted with hexane containing increasing amounts of ethyl acetate. Fractions were monitored by TLC using precoated silica gel plates (Merck silica gel 60 F254, 0.25 mm thick) and the spots were visualized under UV (254 and 366 nm) followed by spraying with Panchal-D (Ceric ammonium sulphate (3.6 g), ammonium hepta molybdate (4.2g), Con. H2SO4 (6.2 ml) in 100 ml of water) is a spraying reagent for TLC and vanillin–sulfuric acid spray reagent. The following ratios of mobile phase were used for fractionation: hexane (100%); hexane and ethyl acetate (95:5); hexane and ethyl acetate (90:10); hexane and ethyl acetate (85:15); hexane and ethyl acetate (80:20); hexane and ethyl acetate (75:25). Six fractions were collected and concentrated; fraction five showed a single spot on TLC which was found to be a pure compound.
The structure of the compound was elucidated by spectroscopic methods such as 1H NMR, 13C-NMR and IR-spectrophotometer.
Establishment of insect culture
Helicoverpa armigera and S. litura larvae were collected from vegetable fields in and around Chennai, India. H. armigera larvae were fed with artificial diet (distilled water [720 ml], chickpea [200 g], sorbic acid [1 g], L-ascorbic acid [3 g], methyl parabane sodium salt [2 g], streptomycin [0.01 g], formaldehyde [40% in 1 ml], agar [12 g], yeast [30 g] and multivitamin drops) and S. litura larvae were fed with castor leaves in the laboratory at 28 ± 1°C, 11 ± 1 h photoperiod and 65–70% r.h. Adults were released into the oviposition chambers for egg laying and provided with 10% honey solution. Cotton and castor leaves were kept inside the cages to facilitate egg laying. Eggs were collected, kept separately and newly hatched larvae were maintained on artificial diet and castor leaves for H. armigera and S. litura, respectively. Freshly moulted 4th instar larvae were used for the experiments.
Antifeedant activity
Antifeedant activity of the crude extracts, fractions and pure compound was studied using the leaf disc no-choice method (Isman et al. 1990). Fresh leaf discs (4-cm diam) of cotton and castor were used for H. armigera and S. litura, respectively. For antifeedant bioassay, the leaf discs dipped in crude extracts at 1%, in fractions at 1000 ppm and in the isolated compound at 100, 250, 500, 1000 and 2000 ppm concentrations, were screened. Leaf discs treated with acetone were used as a negative control. Leaf discs treated with 1% aqueous neem seed kernel extract (NSKE) and azadirachtin 1000 ppm (40.86% purity, obtained from EID-Parry, India Ltd., Chennai) were used as positive controls for crude extracts and the isolated compound, respectively. Wet filter paper was placed in each petri dish (1.5 cm × 9 cm) to avoid early drying of the leaf disc. A single 4th instar larva of H. armigera and S. litura was introduced individually. Five replicates were maintained for each treatment with five larvae per replicate. The experiment was conducted under laboratory conditions (27 ± 2°C) with 14L:10D photoperiod and 75 ± 5% r.h. Progressive consumption of leaf area by the larva was recorded after 24 h in control and treated discs using a leaf area meter (Delta–T Devices, Serial No. 15736 F 96, UK). The percentage of antifeedant activity was calculated according to the formula of Isman et al. (1990).
Statistical analysis
The antifeedant activity data were subjected to one-way analysis of variance. Means with significant variance were separated using Duncan’s Multiple Range F-test. Statistics were performed using SPSS package 11.5 version.
Results
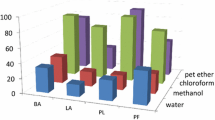
The antifeedant activity of different crude extracts, different fractions and compounds of P. daemia was studied using the leaf disc no-choice method. The crude ethyl acetate extract of P. daemia leaves showed antifeedant activity against H. armigera and S. litura. All crude extracts showed antifeedant activity against 4th instar larvae of H. armigera (F-value: 139.95; P < 0.05) and S. litura (F-value: 50.36; P < 0.05) at 1% concentration with significant differences among solvents used (Table 1). In H. armigera and S. litura, the antifeedant activities of ethyl acetate extract were recorded as 70.3% and 71.82%, respectively; these were statistically significant (P < 0.05) compared with other solvent extracts. Chloroform extract recorded 54.0% antifeedant activity against H. armigera and 48.2% against S. litura; hexane extract recorded 27.8% antifeedant activity against H. armigera and 19.2% against S. litura, suggesting that there was no difference between insect susceptibility among hexane and chloroform extracts. The NSKE aqueous extract treatment showed significant antifeedant activity against H. armigera (77.1%) and against S. litura (73.3%). There was not much variation in antifeedant activity of ethyl acetate extract and NSKE.
The effective ethyl acetate crude extract was subjected to column chromatography. Among the six fractions obtained, fraction 5 had the highest antifeedant activity against both insects tested (H. armigera [75.84%] and S. litura [66.33%]) (Table 2). There was no difference between insect susceptibility except for fractions 5 and 6. Fraction 1 showed poor antifeedant activity against both the insects tested. The F-values were 42.90 for H. armigera and 25.87 for S. litura.
Fraction 5 collected at the mobile phase ratio of 80:20 (hexane: ethyl acetate) was a single compound. The purity was further checked by thin layer chromatography (Merck silica gel 60 F254, 0.25 mm thick) using hexane:ethyl acetate (4:1) mobile phase. When this pure compound was subjected to IR, 1H and 13C NMR spectral analyses, the following results were obtained.
1H NMR (200 MHZ, CDCl3): δ1.30 (m, 2H): δ1.36-1.70 (m-2H), δ1.44-1.48 (m, 2H); δ1.68-170 (m, 2H); δ2.16 (t, 2H); δ1.3-7. 41 (m, 1H); δ1.60-1.67 (m, 2H); δ 3.14 (broad singlet, 1-0H); δ3.40 (broad singlet 1-OH); δ6.42 (s, 1H); 6.50-6.72 (dd, 2H). 13C NMR other carbon appear at 190 (C-12). The IR spectrum showed values of 546.0; 850.0; 952.10; 1108; 1296.6; 1351.7; 1408.4; 1455.0; 1724.30; 1792.40; 2875.0; 3440.5 (broad signal due to –OH group). The above spectral data confirmed the compound to be 6-(4, 7 hydroxy-heptyl) cyclohex-2-ene-1,4-dione) (Fig. 1).
Investigation of the antifeedant activity with the isolated compound showed a maximum antifeedant activity at 2000 ppm concentration against both H. armigera (80.22%) (F-value 47.26) and S. litura (68.31%) (F-value 89.60) (Table 3). Concentrations of 500 and 1000 ppm recorded antifeedant activities of 76.13% and 78.64%, respectively, against H. armigera and 66.4% and 63.7%, respectively, against S. litura larvae. A comparison with azadirachtin showed antifeedant activity of 86.33% against H. armigera and 79.88% against S. litura at 1000 ppm concentration.
Discussion
In the present investigation, ethyl acetate extract of P. daemia showed antifeedant activity of 70.3% and 71.82% against H. armigera and S. litura, respectively. Earlier, Jeyasankar et al. (2010) reported that ethyl acetate extract of Syzygium lineare showed antifeedant activity of 79.4% against S. litura at 5% concentration. Ethyl acetate extract of Couroupita guianensis showed promising antifeedant activity of 66.68% and 69.70% against H. armigera at 2.5% and 5% concentrations, respectively (Baskar et al. 2010). Bagavan et al. (2009) studied the larvicidal activity of hexane, chloroform and ethyl acetate extracts of P. daemia against tick, fluke and mosquitoes. They observed that ethyl acetate extract showed 100% mortality against Anopheles subpictus and Culex tritaeniorhynchus at 1000 ppm concentration and 76% and 71% mortality against Haemaphysalis bispinosa and Paramphistomum cervi, respectively, at 2500 ppm concentrations. Earlier, Muthu et al. (2010) reported that ethyl extract of Atalantia monophylla exhibited 78.67% antifeedant activity against Earias vittella.
In the present investigation, fractions eluted with the hexane:ethyl acetate combination showed antifeedant activity. Fraction 5 showed maximum antifeedant activity of 75.84% and 66.33% against H. armigera and S. litura, respectively, at 1000 ppm concentration. The results of the study agree with the earlier findings of Jeyasankar et al. (2010) who reported that the fractions eluted with hexane: ethyl acetate exhibited maximum antifeedant activity of 91.58% in fraction 6 at 1000 ppm concentration. Many researchers have also reported a similar type of findings (Caasi-Lit and Morallo-Rejesus 1990; Ignacimuthu et al. 2006; Morimoto et al. 2002; Ulrichs et al. 2008)
In our study, 6-(4,7-hydroxy-heptyl) quinone showed statistically significant antifeedant activity against H. armigera and S. litura at almost all the concentrations tested. Most of the potent insect antifeedants are quinoline, indole alkaloids, sesquiterpene lactones, diterpenoids, and triterpenoids (Schoonhoven 1982). Morimoto et al. (1999) reported that quinones such as furoquinones, cyperaquinone and scabequinone, isolated from the basal stem of Cyperus nipponicus and C. distans, exhibited insecticidal properties. Anthraquinone aldehyde isolated from Galium aparine showed promising antifeedant activity against polyphagous pests (Morimoto et al. 2002). A new amino-substituted p-benzoquinone isolated from the methanol extract of roots of Cynanchum wilfordii (Asclepiadaceae) was also insecticidal in nature (Yeo and Kim 1998). Seven quinone compounds (Physcion, Chrysophanol, Emodin, Ventiloquinone, Ventiloquinone B, Ventiloquinone E and Ventilone B) isolated from Ventilago madaraspatana were reported to be insecticidal against S. litura and H. vigintioctopunctata (Coccinellidae). The antifeedant activity of 6-(4,7-hydroxy-heptyl) quinone could be attributed to the presence of long chain aliphatic residue and of primary and secondary alcoholic functional groups. The long aliphatic chain is responsible for a hydrophobic nature; quinone ring improves the polarity character of the molecule. The presence of quinone moiety with secondary alcohol group and aliphatic side chain in the compound made the food unpalatable, with the result that the feeding rate of the larvae was greatly reduced. The isolated compound, 6-(4,7-hydroxy-heptyl) quinone also caused malformation and mortality in larval, pupal and adult stages. This is the first report on the bioactivity of the newly isolated compound from P. daemia and it could possibly be used as a component in biopesticide formulation.
References
Alam, M. Z. (1969). Pest of tomato, Prodenia litura F. Insects of vegetables and their control in East Pakistan. Agricultural Information Service Dacca pp. 113–115.
Bagavan, A., Kamaraj, C., Elango, G., Abduz Zahir, A., & Abdul Rahuman, A. (2009). Adulticidal and larvicidal efficacy of some medicinal plant extracts against tick, fluke and mosquitoes. Veterinary Parasitology, 166, 286–292.
Baskar, K., Maheswaran, R., Kingsley, S., & Ignacimuthu, S. (2010). Bioefficacy of Couroupita guianensis (Aubl.) against Helicoverpa armigera (Hub.) (Lepidoptera: Noctuidae) larvae. Spanish Journal of Agricultural Research, 8, 135–141.
Bowers, B. W., Ohta, T. C., Cleere, J. S., & Marsell, P. A. (1976). Discovery of insect anti-juvenile hormones in plants. Science, 193, 542–547.
Caasi-Lit, M. T., & Morallo-Rejesus, B. (1990). Effects of Aristolachia extracts on the common cutworm Spodoptera litura (F.). Philippine Entomology, 8, 761–769.
Ignacimuthu, S., Maria Packiam, S., Pavunraj, M., & Selvarani, N. (2006). Antifeedant activity of Sphaeranthus indicus L. against Spodoptera litura Fab. Entomon, 31, 41–44.
Isman, B., Koul, O., Lucyzynski, A., & Kaminski, J. (1990). Insecticidal and antifeedant bioactivities of neem oils and their relationship to Azadirachtin content. Journal of Agricultural and Food Chemistry, 38, 1407–1411.
Jeyasankar, A., Raja, N., & Ignacimuthu, S. (2010). Antifeedant and growth inhibitory activities of Syzygium lineare Wall (Myrtaceae) against Spodoptera litura Fab. (Lepidoptera: Noctuidae). Current Research Journal of Biological Sciences, 2, 173–177.
Kabaru, J. M., & Gichia, L. (2001). Insecticidal activity of extracts derived from different parts of the mangrove tree Rhizophora mucronata (Rhizophoraceae) Lam. against three orthropods. African Journals of Science and Technology (AJST), Science and Engineering Series, 2, 44–49.
Katyal, T., & Satake, M. (1996). Environmental pollution. In R. Kumar (Ed.), Introduction to environmental pollution (pp. 1–16). New Delhi, India: Anmol Publications.
Leatemia, J. A., & Isman, M. B. (2004). Insecticidal activity of crude seed extracts of Annona spp., Lanium domesticum and Sandoricum koetjape against lepidopteran larvae. Phytoparasitica, 32, 30–37.
Macleod, J. K., Moeller, P. D. R., Molinski, T. F., & Koul, O. (1990). Antifeedant activity against Spodoptera litura larvae and [13C] NMR spectral assignments of the meliatoxins. Journal of Chemical Ecology, 16, 2511–2518.
Mohammed, S., Kasera, P. K., & Shula, J. K. (2004). Unexploited plants of potential medicinal value from the Indian Thar desert. Natural Product Radiance, 3, 69–74.
Morimoto, M., Fujii, Y., & Komai, K. (1999). Antifeedants in Cyperaceae: Coumaran and quinines from Cyperus spp. Phytochemistry, 51, 605–608.
Morimoto, M., Tanimoto, K., Saktani, A., & Komai, K. (2002). Antifeedant activity of anthraquinone aldehydes in Galium aparine L. against Spodoptera litura F. Phytochemistry, 60, 163–166.
Moussa, M. A., Zaher, M. A., & Kotby, F. (1960). Abundance of cotton leafworm, Prodenia litura F. relation to host plants. I-Host plants and their effect on biology (Lepidoptera: Agrotidae). Bulletin of the Entomological Society of Egypt, 44, 241–251.
Muthu, C., Baskar, K., Kingsley, S., & Ignacimuthu, S. (2010). Bioefficacy of Atalantia monophylla (L.) Correa against Earias vittella Fab. Journal of Central European Agriculture, 11, 23–26.
Nas, M. N. (2004). In vitro studies on some natural beverages as botanical pesticides against Erwinia amylovora and Curobacterium flaccumfaciensis subsp. Poinsettiae. Turkish Journal of Agriculture, 28, 57–61.
Pavunraj, M., Subramanian, K., Muthu, C., Prabu Seenivasan, S., Duraipandiyan, V., Maria Packiam, S., et al. (2006). Bioefficacy of Excoecaria agallocha (L.) leaf extract against armyworm, Spodoptera litura (Fab.) (Lepidoptera: Noctuidae). Entomon, 31, 37–40.
Raja, N., Jeyasankar, A., Jeyakumar, V., & Ignacimuthu, S. (2005). Efficacy of Hyptis suaveolens against lepidopteran pests. Current Science, 88, 220–222.
Ramya, S., & Jayakumararaj, R. (2009). Antifeedant activity of selected ethno-botanicals used by tribals of Vattal Hills on Helicoverpa armigera (Hübner). Journal of Pharmacy Research, 2, 1414–1418.
Roy, B., Amin, R., Uddin, M. N., Islam, A. T. M. S., Islam, M. J., & Halder, B. C. (2005). Leaf extracts of shiyalmutra (Blumea lacera Dc.) as botanical pesticides against lesser grain borer and rice weevil. International Journal of Biological Science, 5, 201–204.
Sakthivadivel, M., & Danial, T. (2008). Evaluation of certain insecticidal plants for the control of vector mosquitoes viz., Culex quinquefasciatus, Anopheles stephensi and Aedes aegypti. Applied Entomology and Zoology, 431, 57–63.
Schmutterer, H. (1995). The neem tree Azadirachta indica A. Juss. and meliaceous plants. Weinheim, Germany: VCH Publishers.
Schoonhoven, L. M. (1982). Biological aspects of antifeedants. Entomologia Experimentalis et Applicata, 31, 57–69.
Stoll, G. (1988). Natural crop protection in the tropics (pp. 11–50). Weikersheim, Germany: Verlag Margraf.
Subramaniyan, S., & Mohankumar, S. (2006). Genetic variability of the bollworm, Helicoverpa armigera, accruing on different host plants. Journal of Insect Science, 6, 26.
Ulrichs, C. H., Mews, I., Adhikary, S., Bhattacharyya, A., & Goswami, A. (2008). Antifeedant activity and toxicity of leaf extracts from Portesia coarctata Takeoka and their effects on the physiology of Spodoptera litura (F.). Journal of Pest Science, 18, 79–84.
Wealth of India (1966). Raw Materials: VII. N-Pe Publications & Information, Directorate, CSIR, New Delhi, India.
Williams, A. L., Mitchell, E. R., Heath, R. R., & Barfield, C. S. (1986). Oviposition deterrents for fall armyworm (Lepidoptera: Noctuidae) from larval frass, corn leaves and artificial diet. Environmental Entomology, 15, 327–330.
Yasui, H., Kato, A., & Yazawa, M. (1998). Antifeedant to armyworm, Spodoptera litura and Pseudaletia separata, from bitter gourd leaves, Momordica charantia. Journal of Chemical Ecology, 24, 803–813.
Yeo, H., & Kim, J. (1998). A benzoquinone from Cynanchum wilfordii. Phytochemistry, 46, 1103–1105.
Yoganarasimhan, S. N. (2000). Medicinal plants of India (Vol. 1). Bangalore, India: Interline Publishing Pvt. Ltd.
Acknowledgments
The authors thank Dr. K. Balakrishna, Scientist, Central Drug Research Institute for Siddha, Chennai, for interpreting the structural data. The authors are grateful to the Entomology Research Institute for financial assistance.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Pavunraj, M., Muthu, C., Ignacimuthu, S. et al. Antifeedant activity of a novel 6-(4,7-hydroxy-heptyl) quinone® from the leaves of the milkweed Pergularia daemia on the cotton bollworm Helicoverpa armigera (Hub.) and the tobacco armyworm Spodoptera litura (Fab.). Phytoparasitica 39, 145–150 (2011). https://doi.org/10.1007/s12600-010-0141-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-010-0141-5