Abstract
Polycyclic aromatic hydrocarbons (PAHs), pervasive organic compounds stemming from combustion processes and industrial activities, have raised significant concerns due to their ubiquitous presence in the environment and potential impact on human health. This review provides a comprehensive overview of the physiological effects of PAHs on diverse human body systems. Extensively studied for their respiratory toxicity, inhalation exposure to PAHs is associated with asthma, bronchitis, and impaired lung function. Moreover, certain PAHs are identified as carcinogens, heightening the risk of lung cancer. The cardiovascular system is also vulnerable to PAH exposure, as evidence suggests their contribution to oxidative stress, inflammation, and endothelial dysfunction, pivotal in cardiovascular disease development. PAHs exhibit endocrine-disrupting properties, influencing hormone levels and disrupting reproductive health, correlating with fertility issues, adverse birth outcomes, and developmental abnormalities. Understanding PAH-induced toxicity mechanisms is crucial for developing mitigation strategies. PAHs can directly interact with cellular components, modulate gene expression, induce oxidative stress, and cause DNA damage, leading to cellular dysfunction and apoptosis. This review underscores the ongoing need for research to fully elucidate the physiological effects of PAH exposure on human health. By synthesizing current knowledge, it aims to raise awareness of potential health risks associated with PAHs and stress the importance of preventive measures to reduce exposure. Ultimately, a comprehensive understanding of PAH-induced physiological impacts will inform the development of effective interventions and policies to safeguard human health in environments where PAH contamination is prevalent.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Polycyclic aromatic hydrocarbons (PAHs) are a diverse group of organic compounds formed through incomplete combustion of organic materials [1]. They are ubiquitously present in the environment and are generated through various anthropogenic activities, such as industrial processes, vehicular emissions, and tobacco smoke [2]. PAHs have garnered significant attention due to their toxicological properties and adverse effects on human health [3, 4].
Over the past decades, extensive research has been conducted to elucidate the impact of PAHs on human physiology [3, 4]. It has become evident that these compounds have far-reaching consequences on various physiological systems in the human body. From the respiratory system to the endocrine system, PAHs exert their influence through diverse mechanisms, including direct interactions with cellular components, modulation of gene expression [5], and disruption of endocrine signaling pathways [6].
The respiratory system represents a prominent target for PAH-induced toxicity. Inhalation exposure to PAHs can lead to respiratory ailments such as asthma, bronchitis, and impaired lung function. Moreover, the carcinogenic potential of certain PAHs has been extensively studied, linking their exposure to an increased risk of lung cancer [7, 8]. Beyond the respiratory system, PAHs have also been implicated in disrupting cardiovascular function. These compounds can induce oxidative stress, inflammation, and endothelial dysfunction, contributing to the development of cardiovascular diseases, including atherosclerosis and hypertension [9, 10]. Furthermore, the endocrine-disrupting properties of PAHs have raised concerns about their impact on reproductive health and developmental outcomes. PAH exposure has been associated with alterations in hormone levels, impaired fertility, adverse birth outcomes, developmental abnormalities [11], and nephrotoxicity [9, 12].
While strides have been made in understanding the diverse physiological impacts of PAHs, a notable research gap persists in the comprehensive synthesis of this knowledge. Existing studies have elucidated the associations between PAH exposure and various health ailments, ranging from respiratory disorders to cardiovascular diseases and reproductive health issues. However, a holistic overview that systematically connects the dots from the sources of PAHs to the manifestation of these health concerns is notably lacking. This review aims to bridge this critical gap by offering a nuanced exploration of the entire trajectory – from the sources and ubiquity of PAHs in the environment to the intricate mechanisms through which they exert their effects on human health. By synthesizing existing findings, we endeavor to provide a cohesive narrative that not only consolidates the current state of knowledge but also illuminates avenues for future research. This comprehensive perspective is essential for guiding future investigations and interventions, ultimately contributing to a more profound understanding of the complex interplay between PAH exposure and human health.
2 Materials and methods
To conduct a comprehensive review, a systematic and rigorous approach was employed. The following materials and methods were utilized to gather relevant information and critically analyse the literature:
2.1 Literature search
A thorough search was conducted across various scientific databases, including PubMed, Google Scholar, and Web of Science. The search terms used included “polycyclic aromatic hydrocarbons,” “PAHs,” “human physiology,” “health effects,” and related keywords. Relevant articles published in peer-reviewed journals were included, with publication dates of 2012–2023.
2.2 Selection criteria
The retrieved articles were screened based on their titles and abstracts for relevance to the topic of interest. Studies focusing on the physiological effects of PAHs in humans were included, while those focusing solely on environmental aspects or nonhuman models were excluded. Review articles, original research papers, and meta-analyses were considered for inclusion.
2.3 Data extraction and analysis
Data relevant to the physiological effects of PAHs on different systems in the human body were extracted from the selected articles. Essential information was recorded, such as study design, sample size, exposure assessment, physiological endpoints measured, and main findings. The total number of downloaded articles was 310. Among them, 50 articles were excluded due to their duplication and the need for more helpful information. Therefore, the total number of articles included in this study was 260. The extracted data were organized and synthesized to identify common themes and patterns across the literature.
2.4 Critical evaluation and synthesis
The findings from the included studies were critically evaluated, considering the study designs, strengths, and limitations. The synthesis of information involved identifying consistent trends, discrepancies, and knowledge gaps in the literature. Emphasis was placed on drawing evidence-based conclusions and providing a balanced overview of the current understanding of PAH-induced physiological effects.
2.5 Brief overview of PAHs
PAHs are organic pollutants that are naturally present in the environment, persisting and posing hazards. These pollutants can be found in surface water, soil, and sediments [13]. The occurrence and concentration of PAHs in the environment are influenced by human activities, such as industrialization and urbanization, and natural events, such as forest fires and volcanic emissions [14].
PAHs exhibit various colours, ranging from colourless to pale yellow solids. They comprise two or more fused aromatic rings, which can be arranged linearly, angularly, or in cluster formations [15]. PAHs are categorized based on the number of aromatic rings they possess. Compounds with 2–3 aromatic rings are considered light, while those with 4–6 are classified as heavy [16].
These compounds have high boiling and melting points and low vapour pressure, making their degradation and metabolism challenging in the natural environment [17]. Low-molecular-weight PAHs are highly toxic and have acute effects, whereas high-molecular-weight PAHs are associated with carcinogenic, teratogenic, and mutagenic effects on living organisms [18].
The hydrophobic nature (water-insolubility) and lipophilic properties (solubility in lipids) are common characteristics of PAHs. As Kronenberg et al. [19] emphasized an increase in molecular weight and the number of fused aromatic rings in PAHs leads to an escalation in their hydrophobicity, subsequently hindering their biodegradation.
PAHs originate from three primary sources: petrogenic, pyrogenic, and biological. Pyrogenic PAHs are formed during the anaerobic combustion of organic compounds at high temperatures (ranging from 350 °C to over 1200 °C). Processes such as coal conversion, combustion of motor/truck fuels, and forest fires contribute to the production of pyrogenic PAHs. Petrogenic PAHs arise from transporting and storing crude oil and its derivatives, oil spills in oceanic and freshwater environments, and leakage from large storage tanks [20]. Biological PAHs are either generated during the degradation of vegetative matter or synthesized by specific plants and bacteria [21].
The United States Environmental Protection Agency (USEPA) has classified 16 primary PAHs, including acenaphthene, benzo[ghi]perylene, chrysene, acenaphthylene, benz[a]anthracene, benzo[b]fluoranthene, anthracene, benzo[k]fluoranthene, benzo[a]pyrene, fluoranthene, indeno[1,2,3-cd]pyrene, naphthalene, phenanthrene, dibenz[a,h]anthracene, fluorene, and pyrene, as reported by Mojiri et al. [22]. Additionally, the International Agency for Research on Cancer has identified seven of these PAHs as carcinogenic, namely, benz[a]anthracene, chrysene, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[a]pyrene, indenol[1,2,2-cd]pyrene, and dibenzo[a,h]anthracene [23]. Among all PAHs, benzo(a)pyrene (BaP) is regarded as the most toxic due to its genotoxic activity.
2.6 Sources of PAHs
2.6.1 PAHs in air
In Pakistan, various types of PAHs, including Nap, Ace, Acy, Flu, Phe, Ant, Flt, Pyr, BaA, Chr, BbF, BkF, BaP, IcdP, Daha, and BghiP, have been identified in both indoor and outdoor air samples. The concentrations of these PAHs differ, with outdoor air recording a concentration of 2.132 ng/m3 and indoor air showing a slightly higher concentration of 2.390 ng/m3. Similarly, in Spain, the PM2.5 fraction of air has been analysed for Nap, Phe, Ant, Flt, Pyr, BaA, Chr, BbF, BkF, BaP, and IcdP, with a mean concentration of 0.79 ng/m3. The Basque Country in Spain exhibited a minimum concentration of 0.79 ng/m3 of PAHs bound with PM2.5, whereas China reported a maximum concentration of 4796.6 ng/m3 of PAHs associated with household air pollution. Table 1 provides a comprehensive overview of the identified PAHs in air samples worldwide.
2.6.2 PAHs in soil
In China, various types of PAHs, including Nap, Ace, Flu, Phe, Ant, Flt, Pyr, Chr, BbF, BkF, BaP, IcdP, Daha, and BghiP, have been identified in agricultural soil. The mean concentration of these PAHs in the soil was recorded at 388 ng/g. Similarly, in the southeastern region of Romania, topsoil samples revealed the presence of Nap, Ace, Flu, Phe, Ant, Flt, Pyr, BaA, Chr, BbF, BkF, BaP, IcdP, Daha, and BghiP, with a mean concentration of 214 ng/g. Forest soil in the rural areas of the Pearl River Delta in southern China exhibited the minimum concentration of PAHs at 35.86 ng/g. In contrast, urban soil in Indianapolis City, USA, displayed a maximum concentration of 36,500 ng/g. Table 2 presents a comprehensive compilation of the identified PAHs in soil samples worldwide.
2.6.3 PAHs in water
In the Pearl River Delta of China, various types of PAHs, including Nap, Ace, Acy, Flu, Phe, Ant, Flt, Pyr, BaA, Chr, BbF, BkF, BaP, IcdP, Daha, and BghiP, have been detected in surface water. During the summer, their concentrations were recorded at 25.63 ng/L; in spring, the concentrations were slightly lower at 25.15 ng/L. Similarly, in Japan, seawater samples have shown the presence of Nap, Ace, Acy, Flu, Phe, Ant, Flt, Pyr, BaA, Chr, BbF, BkF, BaP, IcdP, Daha, and BghiP, with an average concentration of 0.03 ng/L. In Nigeria's Anambra River, the minimum concentration of PAHs was observed at 0.017283 ng/L, whereas South Africa's Mvudi River exhibited the highest concentration at 16,585,000 ng/L. Table 3 provides a comprehensive overview of the identified PAHs in different water samples worldwide.
2.6.4 PAHs in vegetables
In China, various types of PAHs, including Nap, Ace, Acy, Flu, Phe, Ant, Flt, Pyr, 2-BrFle, 1-BrPyr, 9,10-Br2Ant, 7-BrBaA, 9-BrAnt, 9-BrPhe, 9-ClFle, 9-ClPhe, 2-ClAnt, 9-ClAnt, and 9,10-Cl2Ant, have been documented in different vegetables, such as rape, chives Pakchoi, spinach, Chinese cabbage, carrot, radish, and potato. These PAHs exhibit varying concentrations, with PAHs recorded at 3.2551875 ng/g, BrPAHs at 0.296 ng/g, and ClPAHs at 0.3655 ng/g. Similarly, in Iran, PAHs such as BaA, Chr, BbF, BkF, BaP, and Daha have been identified in vegetables, with spinach (2.042 ng/g), lettuce (2.211 ng/g), cabbage (1.143 ng/g), potato (0.589 ng/g), carrot (0.564 ng/g), and radish (0.69 ng/g) displaying different concentrations. Iran exhibits the lowest concentration of PAHs in carrots at 0.564 ng/g, whereas Bangladesh records the highest concentration of 1240 ng/g PAHs in red amaranth. Table 4 presents an overview of the identified PAHs found in vegetables across various regions worldwide.
2.6.5 PAHs in milk
In Spain, a variety of PAHs, including Nap, Ace, Acy, Flu, Phe, Ant, Flt, BaA, Chr, BbF, BkF, BaP, IcdP, Daha, and BghiP, have been detected in human milk samples. The concentrations of these PAHs differ, with colostrum recording a concentration of 2.113 ng/g and mature milk showing a slightly lower concentration of 1.34 ng/g. Similarly, in Ghana, breast milk samples have revealed the presence of Nap, 2MethNaP, 1MethNap, Ace, Acy, Flu, Phe, Ant, Flt, Pyr, BaA, Chr, BbF, BkF, BaP, IcdP, Daha, and BghiP, with a mean concentration of 1105.63 ng/g (lipid weight). Milk powder samples in Sri Lanka exhibited the minimum concentration of PAHs at 0.13 ng/g (dw), while powdered milk in Egypt displayed a maximum concentration of 8200 ng/g of PAHs. Table 5 provides a comprehensive overview of the identified PAHs in milk samples from different regions worldwide.
2.6.6 PAHs in meat
In Denmark, a variety of PAHs, including Ace, Acy, Flu, Phe, Ant, Flt, Pyr, BaA, Chr, BbF, BkF, BjF, BaP, BeP, IcdP, Daha, BghiP, CPP, PER, 5MeCHR, DBalP, DBaeP, DBaiP, and DBahP, have been documented with corresponding concentrations in different meat types. The concentrations were recorded as 79.55 ng/g (beef), 220.4166 ng/g (pork), 99.95 ng/g (chicken), 8.7 ng/g (calf), 59 ng/g (lamb), 243 ng/g (lamb on a stick), 17.2 ng/g (salmon), and 13.8 ng/g (prawn). Similarly, in South Africa, PAHs such as BkF, BaP, IcdP, and BghiP have been reported with concentrations of 1.52415 ng/g (beef), 1.847475 ng/g (pork), and 1.587875 ng/g (chicken), respectively. Table 6 comprehensively summarizes the identified PAHs in meat samples across different regions worldwide.
2.6.7 Chemical nature of PAHs
PAHs are lipophilic chemicals or organic compounds composed of multiple fused aromatic rings and occur naturally in coal tar, fossil fuel combustion, forest fires, and open flame-grilled meats. PAHs are found in cigarette smoke, diesel emissions, asphalt surfacing, tar roofing, and aluminum and coke plants. They are typically formed during incomplete combustion of organic matter [140]. PAHs are mostly colorless, white, or pale yellow solid molecules [69]. Due to their mutagenic and carcinogenic qualities, PAHs are among the most common chemical compounds known to cause cancer. Several of these chemicals are carcinogenic, mutagenic, teratogenic, and genotoxic. They are usually bioaccumulate in soft tissues of animals. High stability and an array of sources contribute to PAHs, which can be generically defined as diagenetic, pyrogenic, or petrogenic [141].
These compounds are not directly carcinogenic and act like synergists. The number of aromatic rings present varies among the hundreds of PAH and similar compounds found and identified. PAH is not a single chemical but a mixture of several PAH components. They range in volatility from the pure gas phase (2-ring) through phase distributed (3,4-ring) and essentially non-volatile (5,6-ring) compounds [142] (Fig. 1). There are two types of PAHs: high-molecular-weight PAHs (HMW PAHs, having four or more aromatic rings) and light-molecular-weight PAHs (LMW PAHs, having 2 or 3 aromatic rings). Depending on their molecular weight, they are released either in the form of particles (HMW PAHs) or as gaseous phases (LMW PAHs) [143]. PAHs have a higher resistance to nucleophilic attack due to their biological persistence, which is caused by the presence of concentrated electrons on aromatic rings (Fig. 1). Higher molecular weight PAHs are more complicated when dissolved molecules because they become more lipophilic and lose their water solubility.
Different polycyclic aromatic hydrocarbons with their chemical structure [146]
Since PAHs are produced by industrial processes, thermal breakdown of organic materials—such as biomass or fossil fuels—and landfill effluents, which have been documented to contain different quantities of PAHs in the US, Sweden, and Poland, there are numerous sources of PAHs [144]. In an aerobic environment, PAHs released into the soil quickly biodegrade; however, because they may be poisonous to microorganisms, they may remain for prolonged in anaerobic environments or at high concentrations. Some of them tend to be adsorbed on the soil surface based on their octanol-carbon coefficient (Koc), volatilize based on their vapor pressure from dry soil or wet soil based on their Henry's law coefficient, and migrate towards groundwater if they are not biodegraded in the soil. After entering groundwater, certain PAHs have the tendency to dissolve in water based on their solubility and octanol–water coefficient (Kow); others have the tendency to volatilize from water based on their solubility and Henry's law constant (H); still, others tend to remain at the top of the water or migrate to the base of the groundwater body [145].
2.6.8 Toxic natures of PAHs
PAHs exhibit significant toxicity, exerting genotoxic, mutagenic, teratogenic, immunotoxic, and carcinogenic effects on microorganisms, laboratory animals, and humans. Their lipophilic and hydrophobic nature necessitates metabolic activation to manifest these adverse effects. The intricate combination of various PAH structures within a mixture enhances their toxicity, often through synergistic interactions [147, 148].
IARC has categorized PAHs into the following four groups [149]:
Group 1: Carcinogenic to humans.
Group 2A: Most likely carcinogenic to humans.
Group 2B: Possibly carcinogenic to humans.
Group 3: Not classifiable as carcinogenic to humans.
The toxicological impacts of PAHs are influenced by various factors, including their source, exposure dose, and duration. Soil-bound PAHs, primarily from biomass burning, present the highest cancer risk to humans. These PAHs enter the bodies of organisms primarily through ingestion, followed by dermal contact and inhalation [61, 150]. The health effects associated with PAH exposure range from acute manifestations, such as eye and skin irritation, inflammation, diarrhea, and confusion, to chronic outcomes, including kidney and liver damage, reproductive system abnormalities, compromised immune function, and respiratory dysfunction. Due to their high lipophilicity, PAHs accumulate readily in organisms' internal organs and adipose tissue, particularly in humans [20].
Among the various PAHs, naphthalene and benzo(a)pyrene (B(a)P) act as direct skin irritants. Naphthalene, a prominent component found in mothballs, can induce the breakdown of red blood cells when inhaled in substantial quantities due to its hemolytic properties. B(a)P exhibits neurotoxic and carcinogenic activities within the human body [21, 151]
PAHs also exert adverse effects on the immune system, although higher concentrations are typically required to induce immunotoxicity rather than carcinogenesis. Exposure to PAHs affects the bone marrow, the central site for immune cell production, maturation, and haematopoiesis. Consequently, structural and functional changes occur in the bone marrow, leading to apoptosis of lymphoid tissue, suppression of B and T cells, tumour development, and hypersensitivity. The primary mechanism underlying these effects involves the specific binding of PAHs to aryl hydrocarbon receptors (AhRs) present in immune cells, triggering the production of immune-toxic oxidative and electrophilic metabolites [151,152,153].
2.7 Toxic effects of PAHs on human health
2.7.1 Effect on the respiratory system
PAHs are a significant class of environmental pollutants known for their adverse health impacts, particularly on the human respiratory system. These compounds, characterized by multiple fused benzene rings, are primarily generated through incomplete combustion of organic matter, including fossil fuels, biomass, and tobacco. Decades of research have underscored the toxicity of soot particles containing PAHs, but recent studies have deepened our understanding of the specific chemical constituents and their mechanisms of harm (Table 7; Fig. 2).
Mass spectrometry analysis has revealed that particles with medium oxidation states contain the highest abundance of oxygen-containing PAHs (OPAHs), such as 1,4-naphthoquinone, 9-fluorenone, and anthranone. These compounds are particularly harmful as they induce oxidative stress in human alveolar basal epithelial cells (A549), upregulating metabolites linked to inflammation and cell damage. Pathway analysis indicates that exposure to these OPAHs disrupts critical cellular functions, including the tricarboxylic acid cycle, cell proliferation, and transcriptional regulation, further highlighting their potential to cause respiratory diseases and cancer (Table 7).
Epidemiological studies corroborate the toxicological findings, linking PAH exposure to increased risks of lung inflammation and cancer. In Dhaka, Bangladesh, for instance, traffic emissions during winter led to significant airway deposition of PM2.5- and PM10-bound PAHs, resulting in heightened lung cancer risk. Similarly, in Benin City, Nigeria, PM2.5-bound PAHs in automobile workshops were found to exceed acceptable risk levels for adults, underlining the occupational hazards in urban environments (Table 7).
At the molecular level, PAHs have been shown to alter the expression of microRNAs (miRNAs), which regulate various physiological processes. Dysregulated miRNA expression due to PAH exposure may serve as biomarkers for respiratory conditions, providing a potential tool for early detection and intervention. Furthermore, urinary metabolites of PAHs, such as 1-hydroxynaphthalene and 2-hydroxyfluorene, have been associated with chronic obstructive pulmonary disease (COPD), emphasizing the systemic effects of these pollutants.
Interestingly, maintaining an antioxidant-rich diet and lifestyle has been shown to mitigate some of the adverse effects of PAHs on lung function. A study utilizing data from the National Health and Nutrition Examination Survey (NHANES) revealed that individuals with higher oxidative balance scores exhibited less severe lung function impairment in response to PAH exposure. This suggests that antioxidants might play a protective role against the oxidative damage induced by PAHs (Table 7).
The public health implications of PAH exposure are profound, especially in densely populated urban areas where vehicular emissions are a major source of these pollutants. The cumulative risk from combined chemical and non-chemical stressors necessitates a comprehensive approach to environmental regulation and pollution control. Bioremediation techniques using microorganisms to degrade PAHs in polluted environments offer a promising strategy to reduce their prevalence and associated health risks (Table 7).
Therefore, all these evidences highlight the multifaceted impact of PAHs on the human respiratory system, from molecular disruptions to significant public health challenges. Continued research and stringent regulatory measures are crucial to mitigate the health risks posed by these pervasive environmental contaminants. Addressing PAH pollution through improved combustion technologies, enhanced emission controls, and lifestyle interventions will be essential in safeguarding respiratory health and reducing the burden of related diseases.
2.7.2 Effect on the cardiovascular system
Exposure to PAHs is pervasive, occurring through inhalation of polluted air, ingestion of contaminated food and water, and dermal contact. Increasing evidence highlights the detrimental impact of PAHs on cardiovascular health through various mechanistic pathways, involving oxidative stress, inflammation, endothelial dysfunction, and dysregulation of lipid metabolism (Fig. 3).
Oxidative stress is a primary mechanism by which PAHs exert cardiovascular toxicity. PAHs undergo metabolic activation via cytochrome P450 enzymes to form reactive oxygen species (ROS) and PAH-DNA adducts. These ROS initiate a cascade of oxidative damage to cellular lipids, proteins, and nucleic acids, leading to cellular dysfunction. Inflammatory responses are subsequently triggered as immune cells, such as macrophages, release pro-inflammatory cytokines like IL-6, IL-8, and TNF-α. Chronic inflammation induced by PAHs contributes to the progression of atherosclerosis, a key underlying cause of cardiovascular diseases (CVDs) (Table 8).
Endothelial cells lining the blood vessels play a critical role in maintaining vascular homeostasis. PAH exposure disrupts endothelial function by decreasing nitric oxide (NO) bioavailability, a potent vasodilator and anti-inflammatory molecule. The reduction in NO is partly due to increased ROS, which scavenge NO, and the induction of endothelial nitric oxide synthase (eNOS) uncoupling. This dysfunction is characterized by impaired vasodilation, increased vascular permeability, and a pro-thrombotic state, all of which facilitate the development of atherosclerotic plaques (Table 8).
PAHs also influence lipid metabolism, contributing to cardiovascular risk. Studies have shown that PAH exposure is associated with elevated levels of total cholesterol, low-density lipoprotein (LDL), and triglycerides. This dyslipidemia promotes lipid accumulation within arterial walls, fostering plaque formation. Additionally, PAHs enhance the expression of scavenger receptors on macrophages, increasing the uptake of oxidized LDL and forming foam cells, which are hallmark features of atherosclerosis (Table 8).
Heart rate variability (HRV), a measure of autonomic nervous system function, is adversely affected by PAH exposure. Research involving coke oven workers indicated that higher PAH levels correlate with reduced HRV, reflecting autonomic imbalance and increased cardiovascular risk. Furthermore, PAHs are linked to elevated blood pressure through mechanisms involving sympathetic nervous system activation and direct vascular effects. Elevated blood pressure and reduced HRV are both significant predictors of cardiovascular morbidity and mortality (Table 8).
Recent findings suggest that red blood cell distribution width (RDW) may mediate the effects of PAHs on cardiovascular outcomes, such as ischemic heart disease (IHD). Higher RDW, indicative of red cell size variability, is associated with inflammation and oxidative stress. Mediation analysis reveals that RDW accounts for a small yet significant portion of the total effect of PAH exposure on IHD, highlighting its potential role in PAH-induced cardiovascular pathology (Table 8).
Mitigating the cardiovascular effects of PAHs involves both reducing exposure and enhancing resilience through lifestyle interventions. Studies have demonstrated that adopting a healthy lifestyle, including regular physical activity; can attenuate PAH-induced cardiovascular damage. Exercise has been shown to reduce oxidative stress, inflammation, and endothelial dysfunction, thereby offering protective effects against PAH toxicity. Additionally, maintaining adequate levels of essential metals like chromium may counteract the negative impact of PAHs on HRV and other cardiovascular parameters (Table 8).
2.7.3 Effect on the endocrine system
Hormones play a crucial role in maintaining the normal functioning of the endocrine system, acting as chemical messengers secreted by various glands in the body. Once released, hormones travel through the bloodstream, either conjugated or bound to carrier proteins, to reach their target cells. Through genomic and nongenomic mechanisms, hormones specifically bind to receptors on target cells, initiating signalling pathways.
In the genomic mechanism, a hormone binds to its receptor and dissociates chaperone proteins associated with it, allowing receptor dimerization. The dimerized receptor then binds to specific "response elements" on DNA, leading to alterations in gene expression. On the other hand, the nongenomic mechanism involves hormone binding to cell surface receptors, initiating intracellular signaling pathways.
However, certain chemicals, such as PAHs or endocrine-disrupting chemicals (EDCs), can disrupt the normal functioning of hormones and adversely impact living organisms. PAHs interfere with hormone synthesis, transport, metabolism, and the binding of hormones to target cell receptors, often mimicking hormone actions inappropriately. They can act as agonists or antagonists of estrogen, primarily disrupting the actions of steroid hormones such as estrogens and androgens. This disruption is attributed to the structural similarity of PAHs to the benzene rings found in steroid and thyroid hormones (TSH, T3, and T4).
EDCs, including PAHs, can cross the placenta, leading to potential health consequences for developing fetuses and infants that may manifest later in adulthood. These consequences include reproductive abnormalities, metabolic programming, an impaired immune system, alterations in adrenocortical function, obesity, diabetes, and cardiovascular diseases [87, 172, 173].
2.7.4 Effect on the thyroid gland
Recent research has revealed a special connection between urinary metabolites of PAHs and thyroid hormone (TSH) levels in children and adolescents. In females, elevated levels of PAH urinary metabolites (1-OH Nap, 2-OH Phe, and 1-OH Pyr) demonstrated a positive correlation with increased levels of T3, as depicted in Fig. 4. Conversely, among males, urinary metabolites (1-OH Phe, 2-OH Phe, and 9-OH Pyr) were associated with decreased levels of free T4 [174, 175].
2.7.5 Effect on the pancreas
Pancreatic β cells express two types of receptors: estrogen receptors (ERs) and androgen receptors (ARs). The ERs consist of two subtypes, ERα and ERβ, which play a critical role in the survival, maturation, protection against apoptosis, and transcription of the insulin gene in β cells. Conversely, AR increases the β cell mass, as depicted in Fig. 4. However, exposure to PAHs leads to a downregulation of pancreatic ER expression and decreased AR activity. Consequently, there is a reduction in β-cell mass and impaired insulin secretion [176,177,178].
2.7.6 Effect on the adrenal gland
The adrenal gland plays a crucial role in functioning the hypothalamic‒pituitary‒adrenal (HPA) axis, which is essential for maintaining human well-being. However, as endocrine disruptors, PAHs interfere with the proper functioning of the HPA axis, as depicted in Fig. 4. This disruption can be attributed to the structural and chemical characteristics of PAHs, including increased blood supply, lipophilicity resulting from an abundance of polyunsaturated fatty acids in the plasma membrane, and the presence of CYP450 enzymes that produce toxic metabolites and free radicals. The adrenal gland cortex produces steroid hormones, such as glucocorticoids, mineralocorticoids, and androgens. One essential glucocorticoid hormone is cortisol. Excessive production of cortisol hormone can lead to Cushing's syndrome (hypercortisolism), while insufficient production can result in Addison's disease. These hormonal imbalances significantly impact human health [179, 180].
2.7.7 Effect on the nervous system
As lipophilic compounds, PAHs possess endocrine-disrupting properties and can easily cross the placenta via passive diffusion. Consequently, they harm fertility and can enter the fetus from the mother's body. PAHs exert genotoxic effects on the developing fetus by undergoing metabolic activation through CYP450 enzymes and forming bulky PAH-DNA adducts. These adducts are associated with reduced birth weight, neural tube defects, decreased head circumference, and congenital heart defects in the developing fetus and newborn, as depicted in Fig. 5.
Due to their lipophilic nature, PAHs can penetrate the blood‒brain barrier and induce changes in neuronal activity and synaptic plasticity within the central nervous system. Their exposure can lead to systemic inflammation. Among PAHs, benzo(a)pyrene (BaP), in conjunction with reactive oxygen species (ROS), triggers apoptosis in various brain regions by disrupting the balance of the antioxidant system. Ultimately, this neuronal cell death contributes to conditions such as attention-deficit hyperactivity disorder (ADHD), learning and memory impairment, dysregulated neurotransmitter regulation, anxiety, and depression [3, 181, 182].
Aβ42, abundant in cerebrospinal fluid, strongly interacts with PAHs, particularly BaP, leading to structural alterations. This disruption results in the deposition of Aβ42, causing neuronal damage and disturbance of electrochemical signals. Collectively, these activities contribute to the development of Alzheimer's disease [183].
2.7.8 Effect on the excretory system
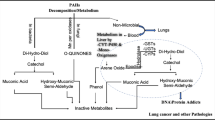
PAHs primarily affect the lungs but also have implications for the liver and kidneys due to their involvement in detoxification and excretion processes. Once absorbed into the body, PAHs undergo rapid detoxification by the liver and are subsequently eliminated through the kidneys in urine, as depicted in Fig. 6. Therefore, the urinary concentrations of PAH metabolites, such as 2-hydroxyphenanthrene (2–OH Phe) and 3-hydroxyphenanthrene (3–OH Phe), serve as biomarkers of PAH exposure [184,185,186].
The level of urinary PAH metabolite shows a positive correlation with serum uric acid levels. Uric acid primarily affects endothelial cells and promotes the production of reactive oxygen species (ROS), leading to increased oxidative stress. Consequently, elevated uric acid levels can impair kidney function and raise the risk of cardiometabolic diseases in children [184, 187].
PAHs transform the liver through three major pathways: (1) the CYP1A1/1B1 and epoxide hydrolase pathway (CYP/EH pathway), (2) the CYP peroxidase pathway, and (3) the aldo–keto reductase pathway (AKR). PAHs, including benzo(a)pyrene (BaP), primarily follow the CYP/EH pathway, leading to the formation of PAH metabolites such as B(a)P-7,8-diol-9,10-epoxides (BPDE). These metabolites can form adducts with DNA and act as carcinogens [188, 189].
Consequently, exposure to PAHs can result in nephrotoxicity and various kidney disorders, including albuminuria, chronic kidney disease (CKD), a decline in estimated glomerular filtration rate (eGFR), kidney stones, and end-stage renal diseases (ESRD) [190,191,192].
Kidney stones have become a prevalent and recurrent disease characterized by the abnormal accumulation of calcium oxalate (CaOx) crystals within the kidneys. The generation of reactive oxygen species (ROS) by PAH metabolites and PAH-DNA adducts is a significant contributing factor. ROS induce oxidative stress in mitochondria, leading to the formation of CaOx crystals, apoptosis, and damage to renal tubular epithelial cells. These apoptotic processes and structural changes in cell membranes facilitate the adhesion of CaOx crystals, initiating a cascade of kidney stone formation [193,194,195].
2.7.9 Effect on male and female reproductive systems
The hypothalamus-pituitary–gonadal (HPG) axis is critical in male and female reproductive processes. The hypothalamus releases GnRH, which stimulates the pituitary gland to secrete follicle-stimulating hormone (FSH) and luteinizing hormone (LH). These hormones act on the testes and ovaries, promoting the production of testosterone, estrogens, and progesterone, which are essential for reproductive functions. However, PAHs have been shown to disrupt these hormones and exhibit endocrine-disrupting properties [147, 196].
As endocrine disruptors, PAHs function as antiestrogens and antiandrogens; they bind to and activate estrogen receptors and aryl hydrocarbon receptors (AhRs). AhR and proteins such as heat shock protein 90 (Hsp90) and p23 reside in the cytoplasm. The binding of PAHs, such as benzo(a)pyrene (BaP), to AhR leads to the formation of an AhR-PAH complex in the cytoplasm, releasing Hsp90. This complex then translocates to the nucleus and binds to xenobiotic response elements (XREs) located in the promoter region of the CYP1A and CYP1B genes. Cytochrome P450 enzymes activate PAHs through monooxygenation, converting them into reactive diols and epoxides, as illustrated in Fig. 7. These reactive intermediates form adducts with DNA, inducing lipid peroxidation, generation of reactive oxygen species (ROS), DNA damage, and ultimately apoptosis.
In males, exposure to PAHs can result in a short telomere length (STL) of sperm DNA, leading to male infertility and poor semen quality. Reproductive dysfunctions such as idiopathic male infertility and cryptorchidism are among the significant consequences of endocrine disruption caused by PAH exposure [197,198,199].
In females, exposure to PAHs can cause menstrual cycle abnormalities, DNA damage in oocytes, ovarian damage, polycystic ovary syndrome, and reproductive diseases. Additionally, exposure to PAHs during pregnancy can result in adverse birth outcomes, including low IQ, birth weight, and premature delivery [151, 200].
The exposure of males to PM2.5 has deleterious effects on the hypothalamic-pituitary axis, spermatogenesis, and fertilization. PM2.5 triggers the production of reactive oxygen species (ROS) in the body, leading to oxidative stress. This oxidative stress initiates damage signals, depletion of antioxidants, and lipid peroxidation of the sperm plasma membrane, ultimately reducing membrane fluidity. These changes ultimately lead to DNA damage, apoptosis, and potential carcinogenesis, as illustrated in Fig. 7. Moreover, oxidative stress diminishes the production of endothelial NO, which plays a critical role in smooth muscle relaxation within the cavernosa. Consequently, PM2.5 impairs the ability to achieve an erection, leading to erectile dysfunction [201,202,203].
In females, exposure to Phe (phenantherene) hampers ovarian development and gives rise to various health issues in offspring. Excessive ROS in the oocyte leads to oxidative stress, disrupting calcium homeostasis and mitochondrial membrane potential. This imbalance affects mitochondrial fusion and fission due to the overexpression of Drp1 and Mfn2. Additionally, aberrant TPX2 expression disrupts spindle assembly and morphology, which is crucial for oocyte meiosis. As a result, these factors collectively impact oocyte maturation [204, 205].
3 Conclusion
In conclusion, this review article provides a comprehensive overview of the impact of PAHs on human physiology. PAHs are ubiquitous environmental pollutants that pose significant risks to human health. The extensive literature examined in this review highlights the detrimental effects of PAH exposure on various physiological systems, including the respiratory, cardiovascular, endocrine, reproductive, renal, and central nervous systems. The respiratory system is particularly vulnerable to PAHs, with evidence linking exposure to respiratory diseases such as asthma, chronic obstructive pulmonary disease (COPD), and lung cancer. PAH-induced inflammation, oxidative stress, and DNA damage contribute to the pathogenesis of these respiratory conditions.
Furthermore, PAHs disrupt cardiovascular homeostasis, leading to endothelial dysfunction, hypertension, and an increased risk of cardiovascular diseases. The mechanisms underlying these effects involve oxidative stress, inflammation, and the disruption of nitric oxide pathways. The endocrine-disrupting properties of PAHs have been extensively documented, affecting hormone synthesis, secretion, and receptor signalling. Dysregulation of the hypothalamic-pituitary–gonadal axis, thyroid function, and adrenal gland activity can lead to reproductive dysfunction, hormonal imbalances, and adverse developmental outcomes. PAH exposure has also been associated with renal toxicity, including nephrotoxicity, albuminuria, chronic kidney disease, and the formation of kidney stones. Oxidative stress and the formation of reactive oxygen species contribute to renal damage and impaired kidney function. PAHs can cross the blood‒brain barrier in the central nervous system and exert neurotoxic effects. They have been linked to neurodevelopmental disorders, cognitive impairments, altered neurotransmitter regulation, and an increased risk of neurodegenerative diseases such as Alzheimer's disease. Overall, the evidence suggests that PAHs have profound implications for human physiology, posing risks to multiple organ systems and contributing to the burden of various diseases. Understanding the mechanisms of these effects is crucial for developing effective preventive strategies and interventions. Given the ubiquity of PAHs in the environment and the potential for exposure through air pollution, occupational settings, and dietary sources, it is imperative to prioritize efforts to reduce PAH emissions, improve monitoring and risk assessment, and implement appropriate regulatory measures. Further research is needed to fill knowledge gaps, particularly regarding the long-term health effects of low-level chronic PAH exposure, the role of genetic susceptibility, and the potential for remediation strategies. Additionally, studies exploring interventions and protective measures to mitigate the adverse effects of PAHs on human physiology are warranted.
Therefore, this review underpins the importance of recognizing PAHs as significant environmental pollutants with profound implications for human health. Enhanced awareness, continued research, and targeted interventions are essential for minimizing exposure, protecting human physiology, and promoting healthier environments for current and future generations.
Data availability
All data collected, analyzed or generated during this study are included in this article.
Abbreviations
- ↑:
-
Increase
- ↓:
-
Decrease
- °C:
-
Degree Celsius
- 3-MECA:
-
3-Methylcholanthrene
- 9-FO:
-
9-Fluorenone
- Ace:
-
Acenaphthene
- ACTH:
-
Adrenocorticotropic hormone
- Acy:
-
Acenaphthylene
- AhRs:
-
Aryl hydrocarbon receptors
- ALT:
-
Alanine transaminase
- AMPK:
-
AMP-activated protein kinase
- Ant:
-
Anthracene
- ARNT:
-
Aryl hydrocarbon receptor nuclear translocator
- ATQ:
-
Anthraquinone
- Azu:
-
Azulene
- Aβ42:
-
Amyloid β42
- BaA:
-
Benzo (a) anthracene
- BaAQ:
-
Benzo[a]anthracene-7,12- dione
- BaP:
-
Benzo (a) pyrene
- BaPO:
-
9,10-Dihydro-8H-benzo[a]pyren-7-one
- BBB:
-
Blood brain barrier
- BbF:
-
Benzo (b) fluoranthene
- BbFLO:
-
11H-Benzo[b]fluorene-11-one
- BcdPO:
-
6H–benzo[cd]pyren-6-one
- BcF:
-
Benzo (c) fluorene
- BcP:
-
Benzo (c) phenanthrene
- BcPhe:
-
Benzo (c) phenanthrene
- BeP:
-
Benzo (e) pyrene
- BghiP:
-
Benzo (ghi) perylene
- BkF:
-
Benzo (k) fluoranthene
- BP:
-
Biphenyl
- BrPAHs:
-
Brominated polycyclic aromatic hydrocarbons
- BZA:
-
7H–benz[de]anthracene-7-one
- Car:
-
Carbazole
- Chr:
-
Chrysene
- Cl:
-
Chloro
- ClPAHs:
-
Chlorinated polycyclic aromatic hydrocarbons
- Cor:
-
Coronene
- CP:
-
Cyclopenta[cd]pyrene
- CPP:
-
Cyclopenta (c,d) pyrene
- CRH:
-
Corticotropin-releasing hormone
- CYP450:
-
Cytochrome P450
- cyt c:
-
Cytochrome c
- DacP:
-
Dibenzo (a,c) pyrene
- DaeF:
-
Dibenzo (a,e) fluoranthene
- DahA:
-
Dibenzo (a,h) anthracene
- DahP:
-
Dibenzo (a,h) pyrene
- DaiP:
-
Dibenzo (a,i) pyrene
- DalP:
-
Dibenzo (a,l) pyrene
- DBF:
-
Dibenzofuran
- DBT:
-
Dibenzo thiopene
- DLLME:
-
Dispersive Liquid‒Liquid Microextraction
- DMBaA:
-
Dimethylbenz (a) anthracene
- DNA:
-
Deoxyribonucleic acid
- Drp1:
-
Dynamin-related protein 1
- dw:
-
Dry weight
- ECD:
-
Electron capture detector
- EDCs:
-
Endocrine disrupting chemicals
- EI:
-
Electron ionization
- ER:
-
Endoplasmic reticulum
- et al.:
-
Et. alibi
- Eth:
-
Ethyl
- FID:
-
Flame ionization detector
- FLD:
-
Fluorescence detectors
- Flt:
-
Fluoranthene
- Flu:
-
Fluorene
- FSHR:
-
Follicle stimulating hormone receptor
- fw:
-
Fresh weight
- GC:
-
Gas chromatography
- GSH:
-
Glutathione
- GST:
-
Glutathione transferases
- H2O2 :
-
Hydrogen peroxide
- HPLC:
-
High-performance liquid chromatography
- HRB:
-
Hai River Basin
- HRMS:
-
High resolution mass spectrometer
- HuRB:
-
Huai River Basin
- HVAAS:
-
High volume active air sampler
- IcdP:
-
Indeno (1,2,3-cd) pyrene
- ICR:
-
Institute of cancer research
- IL1-β:
-
Interleukin1- β
- Ind:
-
Indene
- IQ:
-
Intelligence Quotient
- ITMS:
-
Ion trap mass spectrometer
- LC:
-
Liquid chromatography
- LHR:
-
Luteinizing hormone receptor
- LRB:
-
Liao River Basin
- MePAHs:
-
Methylated polycyclic aromatic hydrocarbons
- Meth:
-
Methyl
- Mfn2:
-
Mitofusin-2
- mg/kg:
-
Milligram per kilogram
- mg/mL:
-
Milligram per milliliter
- MLR:
-
Multiple linear regression
- mM:
-
Millimole
- MS:
-
Mass Spectrometry
- MSD:
-
Mass selective detector
- MSPE:
-
Magnetic solid phase extraction
- MSW:
-
Municipal solid waste
- Nap:
-
Naphthalene
- NAPHQ:
-
Naphthacene-5,12-dione
- NF-kB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- ng/g:
-
Nanogram per gram
- ng/L:
-
Nanogram per liter
- ng/m3 :
-
Nanogram per cubic meter
- nM:
-
Nanomole
- NO:
-
Nitrogen monoxide
- NPAHs:
-
Nitrated polycyclic aromatic hydrocarbons
- O2 :
-
Oxygen
- OHPAHs:
-
Hydroxyalted polycyclic aromatic hydrocarbons
- PAHs:
-
Polycyclic aromatic hydrocarbons
- PCA:
-
Principal component analysis
- PDA:
-
Photo diode array
- Per:
-
Perylene
- Phe:
-
Phenanthrene
- PM:
-
Particulate matter
- PRB:
-
Pearl River Basin
- Pyr:
-
Pyrene
- QqQ:
-
Triple quadrupole
- Ret:
-
Retene
- ROS:
-
Reactive oxygen species
- Sr. No:
-
Serial number
- SRB:
-
Songhua River Basin
- T3 :
-
Triiodothyronine
- T4 :
-
Tetraiodothyronine
- TOF:
-
Time of flight
- TPX2:
-
Targeting protein for xenopus kinesin-like protein 2
- TRH:
-
Thyrotropin-releasing hormone
- TSH:
-
Thyroid stimulating hormone
- UDPGA:
-
Uridin diphospho-glucoronic acid
- UGTs:
-
Uridinglucoronyl transferases
- UHPLC:
-
Ultra high-performance liquid chromatography
- UHT:
-
Ultra heat treated
- UPLC:
-
Ultra-performance liquid chromatography
- USEPA:
-
United States Environmental Protection Agency
- UV:
-
Ultraviolet
- YRB:
-
Yellow River Basin
- YtRB:
-
Yangtze River Basin
- μg/m3 :
-
Microgram per cubic meter
References
Patel AB, Shaikh S, Jain KR, Desai C, Madamwar D. Polycyclic aromatic hydrocarbons: sources, toxicity, and remediation approaches. Front Microbiol. 2020. https://doi.org/10.3389/fmicb.2020.562813.
Srivastava S. Effects of environmental polycyclic aromatic hydrocarbons exposure and pro-inflammatory activity on type 2 diabetes mellitus in US adults. Open J Air Pollut. 2022;11:29–46. https://doi.org/10.4236/ojap.2022.112003.
Olasehinde TA, Olaniran AO. Neurotoxicity of polycyclic aromatic hydrocarbons: a systematic mapping and review of neuropathological mechanisms. Toxics. 2022. https://doi.org/10.3390/toxics10080417.
Qishlaqi A, Beiramali F. Potential sources and health risk assessment of polycyclic aromatic hydrocarbons in street dusts of Karaj urban area, northern Iran. J Environ Heal Sci Eng. 2019;17:1029–44. https://doi.org/10.1007/s40201-019-00417-3.
Paquette AG, Lapehn S, Freije S, MacDonald J, Bammler T, Day DB, et al. Placental transcriptomic signatures of prenatal exposure to hydroxy-polycyclic aromatic hydrocarbons. Environ Int. 2023;172: 107763. https://doi.org/10.1016/j.envint.2023.107763.
Verheyen VJ, Remy S, Govarts E, Colles A, Rodriguez Martin L, Koppen G, et al. Urinary polycyclic aromatic hydrocarbon metabolites are associated with biomarkers of chronic endocrine stress, oxidative stress, and inflammation in adolescents: FLEHS-4 (2016–2020). Toxics. 2021;9:245. https://doi.org/10.3390/toxics9100245.
Låg M, Øvrevik J, Refsnes M, Holme JA. Potential role of polycyclic aromatic hydrocarbons in air pollution-induced non-malignant respiratory diseases. Resp Res. 2020. https://doi.org/10.1186/s12931-020-01563-1.
Moubarz G, Saad-Hussein A, Shahy EM, Mahdy-Abdallah H, Mohammed AMF, Saleh IA, et al. Lung cancer risk in workers occupationally exposed to polycyclic aromatic hydrocarbons with emphasis on the role of DNA repair gene. Int Arch Occup Environ Health. 2023;96:313–29. https://doi.org/10.1007/s00420-022-01926-9.
Mallah MA, Mallah MA, Liu Y, Xi H, Wang W, Feng F, et al. Relationship between polycyclic aromatic hydrocarbons and cardiovascular diseases: a systematic review. Front Public Health. 2021. https://doi.org/10.3389/fpubh.2021.763706.
Rojas GA, Saavedra N, Morales C, Saavedra K, Lanas F, Salazar LA. Modulation of the cardiovascular effects of polycyclic aromatic hydrocarbons: physical exercise as a protective strategy. Toxics. 2023. https://doi.org/10.3390/toxics11100844.
Padmanabhan V, Song W, Puttabyatappa M. Praegnatio perturbatio—impact of endocrine-disrupting chemicals. Endocrine Rev. 2021. https://doi.org/10.1210/endrev/bnaa035.
Rahman HH, Niemann D, Munson-McGee SH. Association of chronic kidney disease with exposure to polycyclic aromatic hydrocarbons in the US population. Environ Sci Pollut Res. 2022;29:24024–34. https://doi.org/10.1007/s11356-021-17479-2.
Yan J, Kim M, Haberl M, Kwok H, Brunswick P, Macinnis C, et al. Determination of polycyclic aromatic hydrocarbons in surface water using simplified liquid-liquid micro-extraction and pseudo-MRM GC/MS/MS. Anal Methods. 2018;10:405–16. https://doi.org/10.1039/c7ay01902e.
Amirdivani S, Khorshidian N, Ghobadi Dana M, Mohammadi R, Mortazavian AM, Quiterio de Souza SL, et al. Polycyclic aromatic hydrocarbons in milk and dairy products. Int J Dairy Technol. 2019. https://doi.org/10.1111/1471-0307.12567.
Pogorzelec M, Piekarska K. Application of semipermeable membrane devices for long-term monitoring of polycyclic aromatic hydrocarbons at various stages of drinking water treatment. Sci Total Environ. 2018;631–632:1431–9. https://doi.org/10.1016/j.scitotenv.2018.03.105.
Purcaro G, Moret S, Conte LS. Overview on polycyclic aromatic hydrocarbons: occurrence, legislation and innovative determination in foods. Talanta. 2013. https://doi.org/10.1016/j.talanta.2012.10.041.
Zhang Y, Yuan L, He S, Tao H, Xie W, Zhang X, et al. Contemporary research progress on the detection of polycyclic aromatic hydrocarbons. Int J Environ Res Public Health. 2022. https://doi.org/10.3390/ijerph19052790.
Hussain K, Balachandran S, Hoque RR. Sources of polycyclic aromatic hydrocarbons in sediments of the Bharalu river, a tributary of the River Brahmaputra in Guwahati, India. Ecotoxicol Environ Saf. 2015;122:61–7. https://doi.org/10.1016/j.ecoenv.2015.07.008.
Kronenberg M, Trably E, Bernet N, Patureau D. Biodegradation of polycyclic aromatic hydrocarbons: using microbial bioelectrochemical systems to overcome an impasse. Environ Pollut. 2017. https://doi.org/10.1016/j.envpol.2017.08.048.
Abdel-Shafy HI, Mansour MSM. A review on polycyclic aromatic hydrocarbons: source, environmental impact, effect on human health and remediation. Egypt J Petrol. 2016. https://doi.org/10.1016/j.ejpe.2015.03.011.
Khan A, Jahego AR. Polycyclic aromatic hydrocarbons and neurological disorders: from exposure to preventive interventions. Springer, Cham; 2021. pp. 335–353. https://doi.org/10.1007/978-3-030-66376-6_15
Mojiri A, Zhou JL, Ohashi A, Ozaki N, Kindaichi T. Comprehensive review of polycyclic aromatic hydrocarbons in water sources, their effects and treatments. Sci Total Environ. 2019. https://doi.org/10.1016/j.scitotenv.2019.133971.
Zheng B, Wang L, Lei K, Nan B. Distribution and ecological risk assessment of polycyclic aromatic hydrocarbons in water, suspended particulate matter and sediment from Daliao river estuary and the adjacent area, China. Chemosphere. 2016;149:91–100. https://doi.org/10.1016/j.chemosphere.2016.01.039.
Hamid N, Syed JH, Junaid M, Mahmood A, Li J, Zhang G, et al. Elucidating the urban levels, sources and health risks of polycyclic aromatic hydrocarbons (PAHs) in Pakistan: implications for changing energy demand. Sci Total Environ. 2018;619–620:165–75. https://doi.org/10.1016/j.scitotenv.2017.11.080.
Oleagoitia MBZ, Manterola AL, Maurolagoitia JI, de Dicastillo MDML, Álvarez J, Barandiaran MA, et al. Polycyclic aromatic hydrocarbons (PAHs) in air associated with particles PM 2.5 in the Basque Country (Spain). Air Qual Atmos Heal. 2019;12:107–14. https://doi.org/10.1007/s11869-018-0635-8.
Krugly E, Martuzevicius D, Sidaraviciute R, Ciuzas D, Prasauskas T, Kauneliene V, et al. Characterization of particulate and vapor phase polycyclic aromatic hydrocarbons in indoor and outdoor air of primary schools. Atmos Environ. 2014;82:298–306. https://doi.org/10.1016/j.atmosenv.2013.10.042.
Ambade B, Kumar A, Sahu LK. Characterization and health risk assessment of particulate bound polycyclic aromatic hydrocarbons (PAHs) in indoor and outdoor atmosphere of Central East India. Environ Sci Pollut Res. 2021;28:56269–80. https://doi.org/10.1007/s11356-021-14606-x.
Lakhani A. Source apportionment of particle bound polycyclic aromatic hydrocarbons at an industrial location in Agra. India Sci World J. 2012. https://doi.org/10.1100/2012/781291.
Kaur S, Senthilkumar K, Verma VK, Kumar B, Kumar S, Katnoria JK, et al. Preliminary analysis of polycyclic aromatic hydrocarbons in air particles (PM10) in Amritsar, India: sources, apportionment, and possible risk implications to humans. Arch Environ Contam Toxicol. 2013;65:382–95. https://doi.org/10.1007/s00244-013-9912-6.
Kumar A, Ambade B, Sankar TK, Sethi SS, Kurwadkar S. Source identification and health risk assessment of atmospheric PM2.5-bound polycyclic aromatic hydrocarbons in Jamshedpur, India. Sustain Cities Soc. 2020;52:101801. https://doi.org/10.1016/j.scs.2019.101801.
Masih J, Singhvi R, Kumar K, Jain VK, Taneja A. Seasonal variation and sources of polycyclic aromatic hydrocarbons (PAHs) in indoor and outdoor air in a semi arid tract of Northern India. Aerosol Air Qual Res. 2012;12:515–25. https://doi.org/10.4209/aaqr.2011.11.0192.
Sampath S, Shanmugam G, Selvaraj KK, Ramaswamy BR. Spatio-temporal distribution of polycyclic aromatic hydrocarbons (PAHs) in atmospheric air of Tamil Nadu, India, and human health risk assessment. Environ For. 2015;16:76–87. https://doi.org/10.1080/15275922.2014.991002.
Yadav A, Behera SN, Nagar PK, Sharma M. Spatio-seasonal concentrations, source apportionment and assessment of associated human health risks of pm2.5-bound polycyclic aromatic hydrocarbons in Delhi, India. Aerosol Air Qual Res. 2020;20:2805–25. https://doi.org/10.4209/aaqr.2020.04.0182.
Gune MM, Ma WL, Sampath S, Li W, Li YF, Udayashankar HN, et al. Occurrence of polycyclic aromatic hydrocarbons (PAHs) in air and soil surrounding a coal-fired thermal power plant in the south-west coast of India. Environ Sci Pollut Res. 2019;26:22772–82. https://doi.org/10.1007/s11356-019-05380-y.
Chuesaard T, Chetiyanukornkul T, Kameda T, Hayakawa K, Toriba A. Influence of biomass burning on the levels of atmospheric polycyclic aromatic hydrocarbons and their nitro derivatives in Chiang Mai, Thailand. Aerosol Air Qual Res. 2014;14:1247–57. https://doi.org/10.4209/aaqr.2013.05.0161.
Błaszczyk E, Rogula-Kozłowska W, Klejnowski K, Fulara I, Mielżyńska-Švach D. Polycyclic aromatic hydrocarbons bound to outdoor and indoor airborne particles (PM25) and their mutagenicity and carcinogenicity in Silesian kindergartens, Poland. Air Qual Atmos Heal. 2017;10:389–400. https://doi.org/10.1007/s11869-016-0457-5.
Albuquerque M, Coutinho M, Borrego C. Long-term monitoring and seasonal analysis of polycyclic aromatic hydrocarbons (PAHs) measured over a decade in the ambient air of Porto, Portugal. Sci Total Environ. 2016;543:439–48. https://doi.org/10.1016/j.scitotenv.2015.11.064.
Ali N. Polycyclic aromatic hydrocarbons (PAHs) in indoor air and dust samples of different Saudi microenvironments; health and carcinogenic risk assessment for the general population. Sci Total Environ. 2019;696: 133995. https://doi.org/10.1016/j.scitotenv.2019.133995.
Niu M, Ying Y, Bartell PA, Harvatine KJ. The effects of feeding rations that differ in fiber and fermentable starch within a day on milk production and the daily rhythm of feed intake and plasma hormones and metabolites in dairy cows. J Dairy Sci. 2017;100:187–98. https://doi.org/10.3168/jds.2016-11129.
Chen Y, Shen G, Huang Y, Zhang Y, Han Y, Wang R, et al. Household air pollution and personal exposure risk of polycyclic aromatic hydrocarbons among rural residents in Shanxi, China. Indoor Air. 2016;26:246–58. https://doi.org/10.1111/ina.12204.
Lu H, Wang S, Wu Z, Yao S, Han J, Tang X, et al. Variations of polycyclic aromatic hydrocarbons in ambient air during haze and non-haze episodes in warm seasons in Hangzhou, China. Environ Sci Pollut Res. 2017;24:135–45. https://doi.org/10.1007/s11356-016-7303-z.
Cao XF, Liu M, Song YF, Ackland ML. Composition, sources, and potential toxicology of polycyclic aromatic hydrocarbons (PAHs) in agricultural soils in Liaoning, People’s Republic of China. Environ Monit Assess. 2013;185:2231–41. https://doi.org/10.1007/s10661-012-2704-z.
Zhang J, Fan S, Du X, Yang J, Wang W, Hou H. Accumulation, allocation, and risk assessment of polycyclic aromatic hydrocarbons (PAHs) in soil-brassica chinensis system. PLoS ONE. 2015;10: e0115863. https://doi.org/10.1371/journal.pone.0115863.
Chai C, Cheng Q, Wu J, Zeng L, Chen Q, Zhu X, et al. Contamination, source identification, and risk assessment of polycyclic aromatic hydrocarbons in the soils of vegetable greenhouses in Shandong, China. Ecotoxicol Environ Saf. 2017;142:181–8. https://doi.org/10.1016/j.ecoenv.2017.04.014.
Khan S, Cao Q. Human health risk due to consumption of vegetables contaminated with carcinogenic polycyclic aromatic hydrocarbons. J Soils Sediments. 2012;12:178–84. https://doi.org/10.1007/s11368-011-0427-3.
Sun Z, Liu J, Zhuo S, Chen Y, Zhang Y, Shen H, et al. Occurrence and geographic distribution of polycyclic aromatic hydrocarbons in agricultural soils in eastern China. Environ Sci Pollut Res. 2017;24:12168–75. https://doi.org/10.1007/s11356-017-8838-3.
Yang B, Xue N, Zhou L, Li F, Cong X, Han B, et al. Risk assessment and sources of polycyclic aromatic hydrocarbons in agricultural soils of Huanghuai plain, China. Ecotoxicol Environ Saf. 2012;84:304–10. https://doi.org/10.1016/j.ecoenv.2012.07.027.
Liu H, Yu X, Liu Z, Sun Y. Occurrence, characteristics and sources of polycyclic aromatic hydrocarbons in arable soils of Beijing, China. Ecotoxicol Environ Saf. 2018;159:120–6. https://doi.org/10.1016/j.ecoenv.2018.04.069.
Xiao Y, Tong F, Kuang Y, Chen B. Distribution and source apportionment of polycyclic aromatic hydrocarbons (PAHs) in forest soils from urban to rural areas in the Pearl River Delta of southern China. Int J Environ Res Public Health. 2014;11:2642–56. https://doi.org/10.3390/ijerph110302642.
Wang C, Wang X, Gong P, Yao T. Polycyclic aromatic hydrocarbons in surface soil across the Tibetan Plateau: spatial distribution, source and air-soil exchange. Environ Pollut. 2014;184:138–44. https://doi.org/10.1016/j.envpol.2013.08.029.
Zhang S, Yao H, Lu Y, Yu X, Wang J, Sun S, et al. Uptake and translocation of polycyclic aromatic hydrocarbons (PAHs) and heavy metals by maize from soil irrigated with wastewater. Sci Rep. 2017;7:1–11. https://doi.org/10.1038/s41598-017-12437-w.
Zhao L, Hou H, Shangguan Y, Cheng B, Xu Y, Zhao R, et al. Occurrence, sources, and potential human health risks of polycyclic aromatic hydrocarbons in agricultural soils of the coal production area surrounding Xinzhou, China. Ecotoxicol Environ Saf. 2014;108:120–8. https://doi.org/10.1016/j.ecoenv.2014.05.034.
Jia J, Bi C, Guo X, Wang X, Zhou X, Chen Z. Characteristics, identification, and potential risk of polycyclic aromatic hydrocarbons in road dusts and agricultural soils from industrial sites in Shanghai, China. Environ Sci Pollut Res. 2017;24:605–15. https://doi.org/10.1007/s11356-016-7818-3.
Zheng H, Qu C, Zhang J, Talpur SA, Ding Y, Xing X, et al. Polycyclic aromatic hydrocarbons (PAHs) in agricultural soils from Ningde, China: levels, sources, and human health risk assessment. Environ Geochem Health. 2019;41:907–19. https://doi.org/10.1007/s10653-018-0188-7.
Wang Y, Tian Z, Zhu H, Cheng Z, Kang M, Luo C, et al. Polycyclic aromatic hydrocarbons (PAHs) in soils and vegetation near an e-waste recycling site in South China: concentration, distribution, source, and risk assessment. Sci Total Environ. 2012;439:187–93. https://doi.org/10.1016/j.scitotenv.2012.08.018.
Wang Y, He J, Wang S, Luo C, Yin H, Zhang G. Characterisation and risk assessment of polycyclic aromatic hydrocarbons (PAHs) in soils and plants around e-waste dismantling sites in southern China. Environ Sci Pollut Res. 2017;24:22173–82. https://doi.org/10.1007/s11356-017-9830-7.
Jiang Y, Yves UJ, Sun H, Hu X, Zhan H, Wu Y. Distribution, compositional pattern and sources of polycyclic aromatic hydrocarbons in urban soils of an industrial city, Lanzhou. China Ecotoxicol Environ Saf. 2016;126:154–62. https://doi.org/10.1016/j.ecoenv.2015.12.037.
Ma L, Li Y, Yao L, Du H. Polycyclic aromatic hydrocarbons in soil-turfgrass systems in urban Shanghai: contamination profiles, in situ bioconcentration and potential health risks. J Clean Prod. 2021;289: 125833. https://doi.org/10.1016/j.jclepro.2021.125833.
Ene A, Bogdevich O, Sion A. Levels and distribution of organochlorine pesticides (OCPs) and polycyclic aromatic hydrocarbons (PAHs) in topsoils from SE Romania. Sci Total Environ. 2012;439:76–86. https://doi.org/10.1016/J.SCITOTENV.2012.09.004.
Bortey-Sam N, Ikenaka Y, Nakayama SMM, Akoto O, Yohannes YB, Baidoo E, et al. Occurrence, distribution, sources and toxic potential of polycyclic aromatic hydrocarbons (PAHs) in surface soils from the Kumasi metropolis, Ghana. Sci Total Environ. 2014;496:471–8. https://doi.org/10.1016/j.scitotenv.2014.07.071.
Tong R, Yang X, Su H, Pan Y, Zhang Q, Wang J, et al. Levels, sources and probabilistic health risks of polycyclic aromatic hydrocarbons in the agricultural soils from sites neighboring suburban industries in Shanghai. Sci Total Environ. 2018;616–617:1365–73. https://doi.org/10.1016/j.scitotenv.2017.10.179.
Lin C, Liu J, Wang R, Wang Y, Huang B, Pan X. Polycyclic aromatic hydrocarbons in surface soils of Kunming, China: concentrations, distribution, sources, and potential risk. Soil Sediment Contam. 2013;22:753–66. https://doi.org/10.1080/15320383.2013.768201.
Daso AP, Akortia E, Okonkwo JO. Concentration profiles, source apportionment and risk assessment of polycyclic aromatic hydrocarbons (PAHs) in dumpsite soils from Agbogbloshie e-waste dismantling site, Accra, Ghana. Environ Sci Pollut Res. 2016;23:10883–94. https://doi.org/10.1007/s11356-016-6311-3.
Attanayake CP, Hettiarachchi GM, Martin S, Pierzynski GM. Potential bioavailability of lead, arsenic, and polycyclic aromatic hydrocarbons in compost-amended Urban soils. J Environ Qual. 2015;44:930–44. https://doi.org/10.2134/jeq2014.09.0400.
Kumar B, Verma VK, Gaur R, Kumar S, Sharma CS, Akolkar AB. Validation of HPLC method for determination of priority polycyclic aromatic hydrocarbons (PAHS) in waste water and sediments, Pelagia. Res Libr Adv Appl Sci Res. 2014;5:201–9.
Kumar B, Verma VK, Kumar S, Sharma CS. Probabilistic health risk assessment of polycyclic aromatic hydrocarbons and polychlorinated biphenyls in urban soils from a tropical city of India. J Environ Sci Heal. 2013;48:1253–63. https://doi.org/10.1080/10934529.2013.776894.
Bhupander K, Gargi G, Richa G, Dev P, Sanjay K, Shekhar SC. Distribution, composition profiles and source identification of polycyclic aromatic hydrocarbons in roadside soil of Delhi. J Environ Earth Sci. 2012;2:10–23.
Chakraborty P, Sampath S, Mukhopadhyay M, Selvaraj S, Bharat GK, Nizzetto L. Baseline investigation on plasticizers, bisphenol A, polycyclic aromatic hydrocarbons and heavy metals in the surface soil of the informal electronic waste recycling workshops and nearby open dumpsites in Indian metropolitan cities. Environ Pollut. 2019. https://doi.org/10.1016/j.envpol.2018.11.010.
Suman S, Sinha A, Tarafdar A. Polycyclic aromatic hydrocarbons (PAHs) concentration levels, pattern, source identification and soil toxicity assessment in urban traffic soil of Dhanbad, India. Sci Total Environ. 2016;545–546:353–60. https://doi.org/10.1016/j.scitotenv.2015.12.061.
Singh DP, Gadi R, Mandal TK. Levels, sources, and toxic potential of polycyclic aromatic hydrocarbons in urban soil of Delhi, India. Hum Ecol Risk Assess. 2012;18:393–411. https://doi.org/10.1080/10807039.2012.652461.
Sarma H, Islam NF, Borgohain P, Sarma A, Prasad MNV. Localization of polycyclic aromatic hydrocarbons and heavy metals in surface soil of Asia’s oldest oil and gas drilling site in Assam, north-east India: implications for the bio-economy. Emerg Contam. 2016;2:119–27. https://doi.org/10.1016/j.emcon.2016.05.004.
Devi NL, Yadav IC, Shihua Q, Dan Y, Zhang G, Raha P. Environmental carcinogenic polycyclic aromatic hydrocarbons in soil from Himalayas, India: implications for spatial distribution, sources apportionment and risk assessment. Chemosphere. 2016;144:493–502. https://doi.org/10.1016/j.chemosphere.2015.08.062.
Bi X, Luo W, Gao J, Xu L, Guo J, Zhang Q, et al. Polycyclic aromatic hydrocarbons in soils from the Central-Himalaya region: distribution, sources, and risks to humans and wildlife. Sci Total Environ. 2016;556:12–22. https://doi.org/10.1016/j.scitotenv.2016.03.006.
Alekseev I, Abakumov E. The content and distribution of trace elements and polycyclic aromatic hydrocarbons in soils of Maritime Antarctica. Environ Monit Assess. 2020;192:1–22. https://doi.org/10.1007/s10661-020-08618-2.
Kwon HO, Choi SD. Polycyclic aromatic hydrocarbons (PAHs) in soils from a multi-industrial city, South Korea. Sci Total Environ. 2014;470–471:1494–501. https://doi.org/10.1016/j.scitotenv.2013.08.031.
Adeyi AA, Oyeleke P. Heavy metals and polycyclic aromatic hydrocarbons in soil from e-waste dumpsites in Lagos and Ibadan, Nigeria. J Heal Pollut. 2017;7:71–84. https://doi.org/10.5696/2156-9614-7.15.71.
Thiombane M, Albanese S, Di Bonito M, Lima A, Zuzolo D, Rolandi R, et al. Source patterns and contamination level of polycyclic aromatic hydrocarbons (PAHs) in urban and rural areas of Southern Italian soils. Environ Geochem Health. 2019;41:507–28. https://doi.org/10.1007/s10653-018-0147-3.
Melnyk A, Dettlaff A, Kuklińska K, Namieśnik J, Wolska L. Concentration and sources of polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs) in surface soil near a municipal solid waste (MSW) landfill. Sci Total Environ. 2015;530–531:18–27. https://doi.org/10.1016/j.scitotenv.2015.05.092.
Ciarkowska K, Gambus F, Antonkiewicz J, Koliopoulos T. Polycyclic aromatic hydrocarbon and heavy metal contents in the urban soils in southern Poland. Chemosphere. 2019;229:214–26. https://doi.org/10.1016/j.chemosphere.2019.04.209.
Li H, Ran Y. Distribution and bioconcentration of polycyclic aromatic hydrocarbons in surface water and fishes. Sci World J. 2012. https://doi.org/10.1100/2012/632910.
Zhang L, Dong L, Ren L, Shi S, Zhou L, Zhang T, et al. Concentration and source identification of polycyclic aromatic hydrocarbons and phthalic acid esters in the surface water of the Yangtze River Delta, China. J Environ Sci. 2012;24:335–42. https://doi.org/10.1016/S1001-0742(11)60782-1.
Qin N, He W, Kong XZ, Liu WX, He QS, Yang B, et al. Distribution, partitioning and sources of polycyclic aromatic hydrocarbons in the water-SPM-sediment system of Lake Chaohu, China. Sci Total Environ. 2014;496:414–23. https://doi.org/10.1016/j.scitotenv.2014.07.045.
Zhi H, Zhao Z, Zhang L. The fate of polycyclic aromatic hydrocarbons (PAHs) and organochlorine pesticides (OCPs) in water from Poyang Lake, the largest freshwater lake in China. Chemosphere. 2015;119:1134–40. https://doi.org/10.1016/j.chemosphere.2014.09.054.
Qiao M, Qi W, Liu H, Qu J. Oxygenated, nitrated, methyl and parent polycyclic aromatic hydrocarbons in rivers of Haihe river system, China: occurrence, possible formation, and source and fate in a water-shortage area. Sci Total Environ. 2014;481:178–85. https://doi.org/10.1016/j.scitotenv.2014.02.050.
Hong WJ, Jia H, Li YF, Sun Y, Liu X, Wang L. Polycyclic aromatic hydrocarbons (PAHs) and alkylated PAHs in the coastal seawater, surface sediment and oyster from Dalian, Northeast China. Ecotoxicol Environ Saf. 2016;128:11–20. https://doi.org/10.1016/j.ecoenv.2016.02.003.
Zhang Y, Zhang L, Huang Z, Li Y, Li J, Wu N, et al. Pollution of polycyclic aromatic hydrocarbons (PAHs) in drinking water of China: composition, distribution and influencing factors. Ecotoxicol Environ Saf. 2019;177:108–16. https://doi.org/10.1016/j.ecoenv.2019.03.119.
Zhang A, Zhao S, Wang L, Yang X, Zhao Q, Fan J, et al. Polycyclic aromatic hydrocarbons (PAHs) in seawater and sediments from the northern Liaodong Bay. China Mar Pollut Bull. 2016;113:592–9. https://doi.org/10.1016/j.marpolbul.2016.09.005.
Mohammed R, Zhang ZF, Jiang C, Hu YH, Liu LY, Ma WL, et al. Fate and occurrence of polycyclic aromatic hydrocarbons and their derivatives in water and sediment from Songhua river, northeast China. Water. 2021;13:1196. https://doi.org/10.3390/W13091196/S1.
Li P, Cao J, Diao X, Wang B, Zhou H, Han Q, et al. Spatial distribution, sources and ecological risk assessment of polycyclic aromatic hydrocarbons in surface seawater from Yangpu Bay, China. Mar Pollut Bull. 2015;93:53–60. https://doi.org/10.1016/j.marpolbul.2015.02.015.
Lan J, Sun Y, Xiao S, Yuan D. Polycyclic aromatic hydrocarbon contamination in a highly vulnerable underground river system in Chongqing, Southwest China. J Geochemical Explor. 2016;168:65–71. https://doi.org/10.1016/j.gexplo.2016.05.013.
Yu H, Liu Y, Han C, Fang H, Weng J, Shu X, et al. Polycyclic aromatic hydrocarbons in surface waters from the seven main river basins of China: spatial distribution, source apportionment, and potential risk assessment. Sci Total Environ. 2021. https://doi.org/10.1016/j.scitotenv.2020.141764.
Wu M, Xia Z, Zhang Q, Yin J, Zhou Y, Yang H. Distribution and health risk assessment on dietary exposure of polycyclic aromatic hydrocarbons in vegetables in Nanjing, China. J Chem. 2016. https://doi.org/10.1155/2016/1581253.
Yang H, Li J, Lu X, Xi G, Yan Y. Reliable synthesis of bismuth nanoparticles for heavy metal detection. Mater Res Bull. 2013;48:4718–22. https://doi.org/10.1016/j.materresbull.2013.08.008.
Wang Y, Zhang S, Cui W, Meng X, Tang X. Polycyclic aromatic hydrocarbons and organochlorine pesticides in surface water from the Yongding river basin, China: Seasonal distribution, source apportionment, and potential risk assessment. Sci Total Environ. 2018;618:419–29. https://doi.org/10.1016/j.scitotenv.2017.11.066.
Iwekumo A, Oghenekohwiroro E, Godwin A, Solomon O, Uwem B. Source predictions of polycyclic aromatic hydrocarbon (PAHs) concentration in water, sediment, and biota (FISHES) from Ethiope river, Delta State, Southern Nigeria. J Ecol Nat Environ. 2020;12:140–9. https://doi.org/10.5897/jene2020.0832.
Obiakor MO, Okonkwo JC, Ezeonyejiaku CD, Okonkwo CN. Polycyclic aromatic hydrocarbons (PAHs) in freshwater media : factorial effects and human dietary exposure risk assessment. Resour Environ. 2014;4:247–59.
Ekere NR, Yakubu NM, Oparanozie T, Ihedioha JN. Levels and risk assessment of polycyclic aromatic hydrocarbons in water and fish of Rivers Niger and Benue confluence Lokoja, Nigeria. J Environ Heal Sci Eng. 2019;17:383–92. https://doi.org/10.1007/s40201-019-00356-z.
Olayinka OO, Adewusi AA, Olarenwaju OO, Aladesida AA. Concentration of polycyclic aromatic hydrocarbons and estimated human health risk of water samples Around Atlas Cove, Lagos, Nigeria. J Heal Pollut. 2018;8: 109685. https://doi.org/10.5696/2156-9614-8.20.181210.
Nekhavhambe TJ, van Ree T, Fatoki OS. Determination and distribution of polycyclic aromatic hydrocarbons in rivers, surface runoff, and sediments in and around Thohoyandou, Limpopo province, South Africa. Water SA. 2014;40:415–24. https://doi.org/10.4314/wsa.v40i3.4.
Adeniji AO, Okoh OO, Okoh AI. Distribution pattern and health risk assessment of polycyclic aromatic hydrocarbons in the water and sediment of Algoa Bay, South Africa. Environ Geochem Health. 2019;41:1303–20. https://doi.org/10.1007/s10653-018-0213-x.
Edokpayi JN, Odiyo JO, Popoola OE, Msagati TAM. Determination and distribution of polycyclic aromatic hydrocarbons in rivers, sediments and wastewater effluents in Vhembe district, South Africa. Int J Environ Res Public Health. 2016;13:387. https://doi.org/10.3390/ijerph13040387.
Seopela MP, McCrindle RI, Combrinck S, Augustyn W. Occurrence, distribution, spatio-temporal variability and source identification of n-alkanes and polycyclic aromatic hydrocarbons in water and sediment from Loskop dam, South Africa. Water Res. 2020;186: 116350. https://doi.org/10.1016/j.watres.2020.116350.
Montuori P, Aurino S, Garzonio F, Sarnacchiaro P, Nardone A, Triassi M. Distribution, sources and ecological risk assessment of polycyclic aromatic hydrocarbons in water and sediments from Tiber river and estuary, Italy. Sci Total Environ. 2016;566–567:1254–67. https://doi.org/10.1016/J.SCITOTENV.2016.05.183.
Karyab H, Yunesian M, Nasseri S, Mahvi AH, Ahmadkhaniha R, Rastkari N, et al. Polycyclic aromatic hydrocarbons in drinking water of Tehran. Iran J Environ Heal Sci Eng. 2013;11:1–7. https://doi.org/10.1186/2052-336X-11-25.
Sheikh Fakhradini S, Moore F, Keshavarzi B, Lahijanzadeh A. Polycyclic aromatic hydrocarbons (PAHs) in water and sediment of Hoor Al-Azim wetland, Iran: a focus on source apportionment, environmental risk assessment, and sediment-water partitioning. Environ Monit Assess. 2019;191:1–18. https://doi.org/10.1007/s10661-019-7360-0.
Agah H, Mehdinia A, Bastami KD, Rahmanpour S. Polycyclic aromatic hydrocarbon pollution in the surface water and sediments of Chabahar bay, Oman Sea. Mar Pollut Bull. 2017;115:515–24. https://doi.org/10.1016/j.marpolbul.2016.12.032.
Aygun SF, Bagcevan B. Determination of polycyclic aromatic hydrocarbons (PAHs) in drinking water of Samsun and it’s surrounding areas, Turkey. J Environ Heal Sci Eng. 2019;17:1205–12. https://doi.org/10.1007/S40201-019-00436-0.
Dhananjayan V, Muralidharan S, Peter VR. Occurrence and distribution of polycyclic aromatic hydrocarbons in water and sediment collected along the harbour line, Mumbai, India. Int J Oceanogr. 2012;2012:1–7. https://doi.org/10.1155/2012/403615.
Sharma BM, Melymuk L, Bharat GK, Přibylová P, Sáňka O, Klánová J, et al. Spatial gradients of polycyclic aromatic hydrocarbons (PAHs) in air, atmospheric deposition, and surface water of the Ganges river basin. Sci Total Environ. 2018;627:1495–504. https://doi.org/10.1016/j.scitotenv.2018.01.262.
Khuman SN, Chakraborty P, Cincinelli A, Snow D, Kumar B. Polycyclic aromatic hydrocarbons in surface waters and riverine sediments of the Hooghly and Brahmaputra rivers in the Eastern and Northeastern India. Sci Total Environ. 2018;636:751–60. https://doi.org/10.1016/j.scitotenv.2018.04.109.
Santos E, Souza MRR, Vilela Junior AR, Soares LS, Frena M, Alexandre MR. Polycyclic aromatic hydrocarbons (PAH) in superficial water from a tropical estuarine system: Distribution, seasonal variations, sources and ecological risk assessment. Mar Pollut Bull. 2018;127:352–8. https://doi.org/10.1016/j.marpolbul.2017.12.014.
Lee YN, Lee S, Kim JS, Kumar Patra J, Shin HS. Chemical analysis techniques and investigation of polycyclic aromatic hydrocarbons in fruit, vegetables and meats and their products. Food Chem. 2019;277:156–61. https://doi.org/10.1016/j.foodchem.2018.10.114.
Jia J, Bi C, Zhang J, Jin X, Chen Z. Characterization of polycyclic aromatic hydrocarbons (PAHs) in vegetables near industrial areas of Shanghai, China: sources, exposure, and cancer risk. Environ Pollut. 2018;241:750–8. https://doi.org/10.1016/j.envpol.2018.06.002.
Sany SBGT. Carcinogenic Polycyclic Aromatic Hydrocarbons in Vegetables Marketed in Mashhad: Levels, Dietary intakes, and Health Risk. J Nutr Fasting Heal. 2021 [cited 14 Aug 2023]. https://jnfh.mums.ac.ir/index.php/50-slideshow/journal/images/journal/article_19289_7eceb2a9bd8b2ab7cd6c2bed9b21f25f.pdf
Bahrami S, Moore F, Keshavarzi B. Evaluation, source apportionment and health risk assessment of heavy metal and polycyclic aromatic hydrocarbons in soil and vegetable of Ahvaz metropolis. Hum Ecol Risk Assess. 2021;27:71–100. https://doi.org/10.1080/10807039.2019.1692300.
Ashraf MW, Taqvi SIH, Solangi AR, Qureshi UA. Distribution and risk assessment of polycyclic aromatic hydrocarbons in vegetables grown in Pakistan. J Chem. 2013. https://doi.org/10.1155/2013/873959.
Abou-Arab A, Abou-Donia MAM, El-Dars FMSE, Ali OIM, Goda HA. Detection of polycyclic aromatic hydrocarbons levels in Egyptian meat and milk after heat treatment by gas chromatography-mass spectrometry. Int J Curr Microbiol Appl Sci. 2014;3:294–305.
Inam E, Ibanga F, Essien J. Bioaccumulation and cancer risk of polycyclic aromatic hydrocarbons in leafy vegetables grown in soils within automobile repair complex and environ in Uyo, Nigeria. Environ Monit Assess. 2016;188:1–9. https://doi.org/10.1007/s10661-016-5695-3.
Tusher TR, Sarker ME, Nasrin S, Kormoker T, Proshad R, Islam MS, et al. Contamination of toxic metals and polycyclic aromatic hydrocarbons (PAHs) in rooftop vegetables and human health risks in Bangladesh. Toxin Rev. 2021;40:736–51. https://doi.org/10.1080/15569543.2020.1767650.
Ashraf MW, Salam A. Polycyclic aromatic hydrocarbons (pahs) in vegetables and fruits produced in Saudi Arabia. Bull Environ Contam Toxicol. 2012;88:543–7. https://doi.org/10.1007/s00128-012-0528-8.
Soceanu A, Dobrinas S, Stanciu G, Popescu V. Polycyclic aromatic hydrocarbons in vegetables grown in urban and rural areas. Environ Eng Manag J. 2014;13:2311–5. https://doi.org/10.30638/eemj.2014.258.
Luzardo OP, Ruiz-Suárez N, Almeida-González M, Henríquez-Hernández LA, Zumbado M, Boada LD. Multi-residue method for the determination of 57 persistent organic pollutants in human milk and colostrum using a QuEChERS-based extraction procedure. Anal Bioanal Chem. 2013;405:9523–36. https://doi.org/10.1007/s00216-013-7377-0.
Asamoah A, Nikbakht Fini M, Essumang DK, Muff J, Søgaard EG. PAHs contamination levels in the breast milk of Ghanaian women from an e-waste recycling site and a residential area. Sci Total Environ. 2019;666:347–54. https://doi.org/10.1016/j.scitotenv.2019.02.204.
Pulkrabova J, Stupak M, Svarcova A, Rossner P, Rossnerova A, Ambroz A, et al. Relationship between atmospheric pollution in the residential area and concentrations of polycyclic aromatic hydrocarbons (PAHs) in human breast milk. Sci Total Environ. 2016;562:640–7. https://doi.org/10.1016/j.scitotenv.2016.04.013.
Oliveira ACC, Vani RAG. Sewage sludge organic fertilizer as a promoter of initial growth of Euterpe edulis, an endangered palm. Int J Recycl Org Waste Agric. 2020;9:161–70. https://doi.org/10.30486/IJROWA.2020.1890190.1020.
Rawash ESA, Mohamed GG, Souaya ER, Khalil LH, El-Chaghaby GA, El-Gammal MH. Distribution and health hazards of polycyclic aromatic hydrocarbons in egyptian milk and dairy-based products. Beverages. 2018;4:63. https://doi.org/10.3390/beverages4030063.
Santonicola S, De Felice A, Cobellis L, Passariello N, Peluso A, Murru N, et al. Comparative study on the occurrence of polycyclic aromatic hydrocarbons in breast milk and infant formula and risk assessment. Chemosphere. 2017;175:383–90. https://doi.org/10.1016/j.chemosphere.2017.02.084.
Dobrinas S, Soceanu A, Popescu V, Coatu V. Polycyclic aromatic hydrocarbons and pesticides in milk powder. J Dairy Res. 2016;83:261–5. https://doi.org/10.1017/S0022029916000169.
Wang L, Liu A, Zhao Y, Mu X, Huang T, Gao H, et al. The levels of polycyclic aromatic hydrocarbons (PAHs) in human milk and exposure risk to breastfed infants in petrochemical industrialized Lanzhou valley, Northwest China. Environ Sci Pollut Res. 2018;25:16754–66. https://doi.org/10.1007/s11356-018-1799-3.
Yan K, Wu S, Gong G, Xin L, Ge Y. Simultaneous determination of typical chlorinated, oxygenated, and european union priority polycyclic aromatic hydrocarbons in milk samples and milk powders. J Agric Food Chem. 2021;69:3923–31. https://doi.org/10.1021/acs.jafc.1c00283.
Goswami P, Wickrama-Arachchige AUK, Yamada M, Ohura T, Guruge KS. Presence of halogenated polycyclic aromatic hydrocarbons in milk powder and the consequence to human health. Toxics. 2022;10:621. https://doi.org/10.3390/toxics10100621.
Turaki Usman A, Abugu HO, Okoye COB. Environmental impact and human health risk assessment of polycyclic aromatic hydrocarbons (pahs) in raw milk from free-ranging cattles in northwest nigeria. J Environ Heal Sci Eng. 2021;19:1523–34. https://doi.org/10.1007/s40201-021-00708-8.
Shariatifar N, Dadgar M, Fakhri Y, Shahsavari S, Moazzen M, Ahmadloo M, et al. Levels of polycyclic aromatic hydrocarbons in milk and milk powder samples and their likely risk assessment in Iranian population. J Food Compos Anal. 2020;85: 103331. https://doi.org/10.1016/j.jfca.2019.103331.
Mahmoudpour M, Mohtadinia J, Ansarin M, Nemati M. Dispersive liquid-liquid microextraction for HPLC-UV determination of PAHs in milk. J AOAC Int. 2016;99:527–33. https://doi.org/10.5740/jaoacint.15-0169.
Duedahl-Olesen L, Aaslyng M, Meinert L, Christensen T, Jensen AH, Binderup ML. Polycyclic aromatic hydrocarbons (PAH) in Danish barbecued meat. Food Control. 2015;57:169–76. https://doi.org/10.1016/j.foodcont.2015.04.012.
Olatunji OS, Fatoki OS, Opeolu BO, Ximba BJ. Determination of polycyclic aromatic hydrocarbons [PAHs] in processed meat products using gas chromatography—flame ionization detector. Food Chem. 2014;156:296–300. https://doi.org/10.1016/j.foodchem.2014.01.120.
Arfaeinia H, Cheshmazar E, Karimyan K, Darvishmotevalli M, Hashemi SE. Data on concentrations of polycyclic aromatic hydrocarbons (PAHs) in roasted and fried chicken – A case study: Bushehr. Iran Data Br. 2018;21:1842–7. https://doi.org/10.1016/j.dib.2018.11.012.
Hokkanen M, Luhtasela U, Kostamo P, Ritvanen T, Peltonen K, Jestoi M. Critical effects of smoking parameters on the levels of polycyclic aromatic hydrocarbons in traditionally smoked fish and meat products in Finland. J Chem. 2018. https://doi.org/10.1155/2018/2160958.
Zastrow L, Speer K, Schwind KH, Jira W. A sensitive GC–HRMS method for the simultaneous determination of parent and oxygenated polycyclic aromatic hydrocarbons in barbecued meat and meat substitutes. Food Chem. 2021;365: 130625. https://doi.org/10.1016/j.foodchem.2021.130625.
Bandeira GC, Meneses HE. Handbook of polycyclic aromatic hydrocarbons: Chemistry, occurrence and health issues. handbook of polycyclic aromatic hydrocarbons: chemistry, occurrence and health issues. 2013. https://cir.nii.ac.jp/crid/1130000795662646912
Mazeas L, Budzinski H. Polycyclic aromatic hydrocarbon 13C/12C ratio measurement in petroleum and marine sediments: application to standard reference materials and a sediment suspected of contamination from the Erika oil spill. J Chromatogr A. 2001;923:165–76. https://doi.org/10.1016/S0021-9673(01)00911-6.
Ifegwu C, Osunjaye K, Fashogbon F, Oke K, Adeniyi A, Anyakora C. Urinary 1-hydroxypyrene as a biomarker to carcinogenic polycyclic aromatic hydrocarbon exposure. Biomark Cancer. 2012;4:65. https://doi.org/10.4137/BIC.S10065.
Chen YP, Zeng Y, Guan YF, Huang YQ, Liu Z, Xiang K, et al. Particle size-resolved emission characteristics of complex polycyclic aromatic hydrocarbon (PAH) mixtures from various combustion sources. Environ Res. 2022;214: 113840. https://doi.org/10.1016/j.envres.2022.113840.
Eggen T, Moeder M, Arukwe A. Municipal landfill leachates: a significant source for new and emerging pollutants. Sci Total Environ. 2010;408:5147–57. https://doi.org/10.1016/j.scitotenv.2010.07.049.
Balint A. Physical, chemical and toxicological properties of polycyclic aromatic hydrocarbons (PAHs) in human exposure assessments to contaminated soil and groundwater. MATEC Web Conf. 2021;342:03016. https://doi.org/10.1051/matecconf/202134203016.
Bresnick E et al. Lists of polycyclic aromatic hydrocarbons—appendix A. Polycyclic Aromatic Hydrocarbons: Evaluation of Sources and Effects. National Academies Press (US); 1983. p. 20. https://www.ncbi.nlm.nih.gov/books/NBK217760/
Bolden AL, Rochester JR, Schultz K, Kwiatkowski CF. Polycyclic aromatic hydrocarbons and female reproductive health: a scoping review. Reprod Toxicol. 2017. https://doi.org/10.1016/j.reprotox.2017.07.012.
Gao P, da Silva E, Hou L, Denslow ND, Xiang P, Ma LQ. Human exposure to polycyclic aromatic hydrocarbons: metabolomics perspective. Environ Int. 2018. https://doi.org/10.1016/j.envint.2018.07.017.
Ghosal D, Ghosh S, Dutta TK, Ahn Y. Current state of knowledge in microbial degradation of polycyclic aromatic hydrocarbons (PAHs): a review. Front Microbiol. 2016. https://doi.org/10.3389/fmicb.2016.01369.
Zheng H, Xing X, Hu T, Zhang Y, Zhang J, Zhu G, et al. Biomass burning contributed most to the human cancer risk exposed to the soil-bound PAHs from Chengdu economic region, western China. Ecotoxicol Environ Saf. 2018;159:63–70. https://doi.org/10.1016/j.ecoenv.2018.04.065.
Rengarajan T, Rajendran P, Nandakumar N, Lokeshkumar B, Rajendran P, Nishigaki I. Exposure to polycyclic aromatic hydrocarbons with special focus on cancer. Asian Pac J Trop Biomed. 2015. https://doi.org/10.1016/S2221-1691(15)30003-4.
Marris CR, Kompella SN, Miller MR, Incardona JP, Brette F, Hancox JC, et al. Polyaromatic hydrocarbons in pollution: a heart-breaking matter. J Physiol. 2020. https://doi.org/10.1113/JP278885.
Burchiel SW, Gao J. Polycyclic aromatic hydrocarbons and the immune system. Encyclopedi Immunotoxicol. 2014. https://doi.org/10.1007/978-3-642-27786-3_1192-4.
Wang L, Wen W, Yan J, Zhang R, Li C, Jiang H, et al. Influence of polycyclic aromatic compounds and oxidation states of soot organics on the metabolome of human-lung cells (A549): implications for vehicle fuel selection. Environ Sci Technol. 2023;57:21593–604. https://doi.org/10.1021/acs.est.3c05228.
Letelier P, Saldías R, Loren P, Riquelme I, Guzmán N. MicroRNAs as potential biomarkers of environmental exposure to polycyclic aromatic hydrocarbons and their link with inflammation and lung cancer. Int J Mol Sci. 2023. https://doi.org/10.3390/ijms242316984.
Shams Solari M, Ashrafi K, Pardakhti A, Hassanvand MS, Arhami M. Meteorological dependence, source identification, and carcinogenic risk assessment of PM25-bound polycyclic aromatic hydrocarbons (PAHs) in high-traffic roadside, urban background, and remote suburban area. J Environ Heal Sci Eng. 2022;20:813–26. https://doi.org/10.1007/s40201-022-00821-2.
Ravanbakhsh M, Yousefi H, Lak E, Ansari MJ, Suksatan W, Qasim QA, et al. Effect of polycyclic aromatic hydrocarbons (PAHs) on respiratory diseases and the risk factors related to cancer. Polycyclic Aromatic Comp. 2023. https://doi.org/10.1080/10406638.2022.2149569.
Famiyeh L, Xu H, Chen K, Tang YT, Ji D, Xiao H, et al. Breathing in danger: unveiling the link between human exposure to outdoor PM2.5-bound polycyclic aromatic hydrocarbons and lung cancer risk in an urban residential area of China. Sci Total Environ. 2024. https://doi.org/10.1016/j.scitotenv.2023.167762.
Alhamdow A, Zettergren A, Kull I, Hallberg J, Andersson N, Ekström S, et al. Low-level exposure to polycyclic aromatic hydrocarbons is associated with reduced lung function among Swedish young adults. Environ Res. 2021. https://doi.org/10.1016/j.envres.2021.111169.
Peng K, Li Z, Gao TR, Lv J, Wang WJ, Zhan P, et al. Polycyclic aromatic hydrocarbon exposure burden: Individual and mixture analyses of associations with chronic obstructive pulmonary disease risk. Environ Res. 2023. https://doi.org/10.1016/j.envres.2023.115334.
Valdez RM, Rivera BN, Chang Y, Pennington JM, Fischer KA, Löhr CV, et al. Assessing susceptibility for polycyclic aromatic hydrocarbon toxicity in an in vitro 3D respiratory model for asthma. Front Toxicol. 2024. https://doi.org/10.3389/ftox.2024.1287863.
Alves CA, Soares M, Figueiredo D, Oliveira H. Effects of particle-bound polycyclic aromatic hydrocarbons and plasticisers from different traffic sources on the human alveolar epithelial cell line A549. Atmos Environ. 2023. https://doi.org/10.1016/j.atmosenv.2023.119736.
Zhang X, Leng S, Qiu M, Ding Y, Zhao L, Ma N, et al. Chemical fingerprints and implicated cancer risks of Polycyclic aromatic hydrocarbons (PAHs) from fine particulate matter deposited in human lungs. Environ Int. 2023. https://doi.org/10.1016/j.envint.2023.107845.
Wu P. Association between polycyclic aromatic hydrocarbons exposure with red cell width distribution and ischemic heart disease: insights from a population-based study. Sci Rep. 2024. https://doi.org/10.1038/s41598-023-50794-x.
Liu K, Bai Y, Wu D, Zhang Z, Liao X, Wu H, et al. Healthy lifestyle and essential metals attenuated association of polycyclic aromatic hydrocarbons with heart rate variability in coke oven workers. Int J Hyg Environ Health. 2024. https://doi.org/10.1016/j.ijheh.2024.114323.
Zhang Y, Wang Y, Zheng H, Materials JW-J of H, 2024 U. Increased mortality risk from airborne exposure to polycyclic aromatic hydrocarbons. J Hazard Mater. 2024; 134714. https://www.sciencedirect.com/science/article/pii/S0304389424012937
Zhang D, Liu X, Tu J, Xiao Q, Han L, Fu J, et al. Mediating role of glucose-lipid metabolism in the association between the increased risk of coronary heart disease and exposure to organophosphate esters, phthalates, and polycyclic aromatic hydrocarbons. Environ Heal. 2024;2:170–9. https://doi.org/10.1021/ENVHEALTH.3C00155.
Simon K, Bartsch N, Schneider L, van de Weijgert V, Hutzler C, Luch A, et al. Polycyclic aromatic hydrocarbon skin permeation efficiency in vitro is lower through human than pigskin and decreases with lipophilicity. Environ Res. 2024. https://doi.org/10.1016/j.envres.2024.119118.
Barros B, Paiva AM, Oliveira M, Alves S, Esteves F, Fernandes A, et al. Baseline data and associations between urinary biomarkers of polycyclic aromatic hydrocarbons, blood pressure, hemogram, and lifestyle among wildland firefighters. Front Public Heal. 2024. https://doi.org/10.3389/fpubh.2024.1338435.
Zhang A, Zhang H, Mi L, Ding L, Jiang Z, Yu F, et al. Diabetes: a potential mediator of associations between polycyclic aromatic hydrocarbon exposure and stroke. Environ Sci Pollut Res. 2024. https://doi.org/10.1007/s11356-024-32324-y.
Qigang N, Afra A, Ramírez-Coronel AA, Turki Jalil A, Mohammadi MJ, Gatea MA, et al. The effect of polycyclic aromatic hydrocarbon biomarkers on cardiovascular diseases. Rev Environ Health. 2023. https://doi.org/10.1515/reveh-2023-0070.
Darbre PD. Endocrine disruption and male reproductive health. Endocrine Disruption Hum Health. 2015. https://doi.org/10.1016/B978-0-12-801139-3.00009-0.
Darbre PD. Overview of air pollution and endocrine disorders. Int J General Med. 2018. https://doi.org/10.2147/IJGM.S102230.
Kelishadi R, Sobhani P, Poursafa P, Amin MM, Ebrahimpour K, Hovsepian S, et al. Is there any association between urinary metabolites of polycyclic aromatic hydrocarbons and thyroid hormone levels in children and adolescents? Environ Sci Pollut Res. 2018;25:1962–8. https://doi.org/10.1007/s11356-017-0577-y.
Jain RB. Association between polycyclic aromatic hydrocarbons and thyroid function among males and females: data from NHANES 2007–2008. Int J Environ Health Res. 2016;26:405–19. https://doi.org/10.1080/09603123.2015.1135311.
Saber N, Bruin JE, O’Dwyer S, Schuster H, Rezania A, Kieffer TJ. sex differences in maturation of human embryonic stem cell-derived β cells in mice. Endocrinology. 2018;159:1827–41. https://doi.org/10.1210/en.2018-00048.
Harada N, Yoda Y, Yotsumoto Y, Masuda T, Takahashi Y, Katsuki T, et al. Androgen signaling expands β-cell mass in male rats and β-cell androgen receptor is degraded under high-glucose conditions. Am J Physiol Endocrinol Metab. 2018;314:E274–86. https://doi.org/10.1152/ajpendo.00211.2017.
Ou K, Song J, Zhang S, Fang L, Lin L, Lan M, et al. Prenatal exposure to a mixture of PAHs causes the dysfunction of islet cells in adult male mice: association with type 1 diabetes mellitus. Ecotoxicol Environ Saf. 2022;239: 113695. https://doi.org/10.1016/j.ecoenv.2022.113695.
Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, et al. EDC-2: the endocrine society’s second scientific statement on endocrine-disrupting chemicals. Endocr Rev. 2015. https://doi.org/10.1210/er.2015-1010.
Egalini F, Marinelli L, Rossi M, Motta G, Prencipe N, Rossetto Giaccherino R, et al. Endocrine disrupting chemicals: effects on pituitary, thyroid and adrenal glands. Endocrine. 2022. https://doi.org/10.1007/s12020-022-03076-x.
Mortamais M, Pujol J, van Drooge BL, Macià D, Martínez-Vilavella G, Reynes C, et al. Effect of exposure to polycyclic aromatic hydrocarbons on basal ganglia and attention-deficit hyperactivity disorder symptoms in primary school children. Environ Int. 2017;105:12–9. https://doi.org/10.1016/j.envint.2017.04.011.
Redza-Dutordoir M, Averill-Bates DA. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta. 2016. https://doi.org/10.1016/j.bbamcr.2016.09.012.
Kaumbekova S, Torkmahalleh MA, Sakaguchi N, Umezawa M, Shah D. Effect of ambient polycyclic aromatic hydrocarbons and nicotine on the structure of Aβ42 protein. Front Environ Sci Eng. 2023;17:1–10. https://doi.org/10.1007/s11783-023-1615-2.
Farzan SF, Chen Y, Trachtman H, Trasande L. Urinary polycyclic aromatic hydrocarbons and measures of oxidative stress, inflammation and renal function in adolescents: NHANES 2003–2008. Environ Res. 2016;144:149–57. https://doi.org/10.1016/j.envres.2015.11.012.
Guo J, Huang Y, Bian S, Zhao C, Jin Y, Yu D, et al. Associations of urinary polycyclic aromatic hydrocarbons with bone mass density and osteoporosis in U.S. adults, NHANES 2005–2010. Environ Pollut. 2018;240:209–18. https://doi.org/10.1016/j.envpol.2018.04.108.
Sun S, Mao W, Tao S, Zou X, Tian S, Qian S, et al. Polycyclic aromatic hydrocarbons and the risk of kidney stones in us adults: an exposure-response analysis of nhanes 2007–2012. Int J Gen Med. 2021;14:2665–76. https://doi.org/10.2147/IJGM.S319779.
Linke AHP, Oberbach A, Jehmlich N, Schlichting N, Schuler G, Adams V. Hyperuricemia induces endothelial dysfunction by increasing reactive oxygen species. Eur Heart J. 2013;34:P601–P601. https://doi.org/10.1093/eurheartj/eht307.p601.
Moorthy B, Chu C, Carlin DJ. Polycyclic aromatic hydrocarbons: From metabolism to lung cancer. Toxicol Sci. 2015. https://doi.org/10.1093/toxsci/kfv040.
Kasala ER, Bodduluru LN, Barua CC, Sriram CS, Gogoi R. Benzo(a)pyrene induced lung cancer: Role of dietary phytochemicals in chemoprevention. Pharmacol Rep. 2015. https://doi.org/10.1016/j.pharep.2015.03.004.
Bowe B, Xie Y, Li T, Yan Y, Xian H, Al-Aly Z. Particulate matter air pollution and the risk of incident CKD and progression to ESRD. J Am Soc Nephrol. 2018;29:218–30. https://doi.org/10.1681/ASN.2017030253.
Lozano M, Trujillo M. Chemical residues in animal food products: an issue of public health. Public health: methodology, environmental and systems issues. Croatia: Intech Open, Rijeka; 2012. pp. 163–175.
Li D, Chen S, Li Q, Chen L, Zhang H, Li H, et al. Caloric restriction attenuates C57BL/6 J mouse lung injury and extra-pulmonary toxicity induced by real ambient particulate matter exposure. Part Fibre Toxicol. 2020;17:1–17. https://doi.org/10.1186/s12989-020-00354-2.
Khan SR, Canales BK, Dominguez-Gutierrez PR. Randall’s plaque and calcium oxalate stone formation: role for immunity and inflammation. Nat Rev Nephrol. 2021. https://doi.org/10.1038/s41581-020-00392-1.
Kizivat T, Smolić M, Marić I, Levak MT, Smolić R, Čurčić IB, et al. Antioxidant pre-treatment reduces the toxic effects of oxalate on renal epithelial cells in a cell culture model of urolithiasis. Int J Environ Res Public Health. 2017;14:109. https://doi.org/10.3390/ijerph14010109.
Thamilselvan V, Menon M, Thamilselvan S. Oxalate at physiological urine concentrations induces oxidative injury in renal epithelial cells: effect of α-tocopherol and ascorbic acid. BJU Int. 2014;114:140–50. https://doi.org/10.1111/bju.12642.
Singh V, Agrawal NK, Verma R, Singh K. HPG axis The central regulator of spermatogenesis and male fertility. In: Singh V, Agrawal NK, Verma R, Singh K, editors. Male infertility understanding, causes and treatment. Singapore: Springer Singapore; 2017. p. 25–36. https://doi.org/10.1007/978-981-10-4017-7_3.
Dohle GR, Colpi GM, Hargreave TB, Papp GK, Jungwirth A, Weidner W. EAU guidelines on male infertility. Eur Urol. 2005;48:703–11. https://doi.org/10.1016/j.eururo.2005.06.002.
Misaki K, Suzuki G, Tue NM, Takahashi S, Someya M, Takigami H, et al. Toxic identification and evaluation of androgen receptor antagonistic activities in acid-treated liver extracts of high-trophic level wild animals from Japan. Environ Sci Technol. 2015;49:11840–8. https://doi.org/10.1021/acs.est.5b02288.
Ling X, Zhang G, Chen Q, Yang H, Sun L, Zhou N, et al. Shorter sperm telomere length in association with exposure to polycyclic aromatic hydrocarbons: results from the MARHCS cohort study in Chongqing, China and in vivo animal experiments. Environ Int. 2016;95:79–85. https://doi.org/10.1016/J.ENVINT.2016.08.001.
Rattan S, Zhou C, Chiang C, Mahalingam S, Brehm E, Flaws JA. Exposure to endocrine disruptors during adulthood: consequences for female fertility. J Endocrinol. 2017. https://doi.org/10.1530/JOE-17-0023.
Albersen M, Orabi H, Lue TF. Evaluation and treatment of erectile dysfunction in the aging male: a mini-review. Gerontology. 2011. https://doi.org/10.1159/000329598.
Li J, Wang Y, Xie X. Cl/Br ratios and chlorine isotope evidences for groundwater salinization and its impact on groundwater arsenic, fluoride and iodine enrichment in the Datong basin, China. Sci Total Environ. 2016;544:158–67. https://doi.org/10.1016/j.scitotenv.2015.08.144.
Wang L, Luo D, Liu X, Zhu J, Wang F, Li B, et al. Effects of PM2.5 exposure on reproductive system and its mechanisms. Chemosphere. 2021. https://doi.org/10.1016/j.chemosphere.2020.128436.
Sun L, Zuo Z, Chen M, Chen Y, Wang C. Reproductive and transgenerational toxicities of phenanthrene on female marine medaka (Oryzias melastigma). Aquat Toxicol. 2015;162:109–16. https://doi.org/10.1016/j.aquatox.2015.03.013.
Niu D, Chen KL, Wang Y, Li XQ, Liu L, Ma X, et al. Hexestrol deteriorates oocyte quality via perturbation of mitochondrial dynamics and function. Front Cell Dev Biol. 2021;9: 708980. https://doi.org/10.3389/fcell.2021.708980.
Funding
The author(s) received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
Sombiri Sombiri gathered all the information, with significant assistance from Namrata Balhara, Deepak Attri, and Isha Kharb during the initial draft preparation. Arup Giri oversaw conceptualization, methodology, writing, review, editing, and supervision.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publications
All authors are agreed for the publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sombiri, S., Balhara, N., Attri, D. et al. An overview on occurrence of polycyclic aromatic hydrocarbons in food chain with special emphasis on human health ailments. Discov Environ 2, 87 (2024). https://doi.org/10.1007/s44274-024-00121-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44274-024-00121-6