Abstract
The liver, the largest solid organ in the body, is susceptible to metabolic diseases and malignant tumors. Studying its physiological and pathological processes helps to optimize the clinical treatment. Organoids are a novel tool for studying physical development, disease mechanisms, and high-throughput drug screening due to their similarity in composition, structure, and function to internal organs. Recent studies have shown that stem cells, hepatocytes, or cholangiocytes can form “liver organoids” under the synergistic action of specific extracellular matrix and various signaling molecules. This review outlines techniques for generating liver organoids that maximally recapitulate the liver structure and functions in vitro and thoroughly discusses the customary applications of organoids derived from liver tissue, induced pluripotent stem cells (iPSCs) and liver tumors. In this review, a meticulous analysis is provided of the comparatively advanced culture systems used in the construction of liver cancer-derived organoids. Additionally, we reviewed the progress of liver organoids in disease modeling, drug efficacy, and toxicity evaluation, in hopes of generating innovative ideas for the research and applications of liver organoids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The liver is the largest metabolic organ, producing bile and proteins and playing a crucial role in immunity, metabolism, and coagulation. Viral infections, heavy drinking, inflammation, metabolic disorders, genetic variations, and malignant tumors can cause serious damage to the liver, leading to severe liver disease and a lethal outcome [1]. Therefore, it is of great value to study the physiological and pathological mechanism of the liver, which contributes to proposing appropriate clinical diagnosis and treatment.
Current studies on liver diseases are mainly conducted on cellular and animal models [2, 3]. Due to the limitations of the traditional two-dimensional (2D) cell culture system, the liver parenchymal cells cultured in vitro usually lack certain liver-specific genes and biological functions, such as apical junction of cells [4, 5]. These systems also lack cell–cell and cell-extracellular matrix (ECM) interactions. Therefore, it is impossible to reproduce the heterogeneity and complex structural characteristics of liver [6, 7]. Animal models of liver disease possess the advantages of functional vascular systems, extracellular matrix, and immune system over in vitro systems. However, animal models, derived from other species, differ in physiology, drug metabolism and phenotypes compared to humans [8].
Recent studies have shown that stem cells or hepatic cancer cells can generate liver organoids using the three-dimensional (3D) cells culture with multiple signal molecules. These cells are split through cell differentiation and self-organization in a limited space, which is similar to the development of liver organs or tumorigenesis [9]. During cultivation, organoids can maintain their structure and function for a long time. Even after multiple passages, many characteristics of the organisms can be maintained in the organoids, with no obvious genetic or physiological changes [10]. Presently, growing studies have reported the construction of organoids, such as the stomach [11, 12] and brain [13, 14]. These organoid models are of great assistance to explore the molecular mechanisms of organ development in vitro. Furthermore, organoid-culture technology also has been widely applied in liver disease studies [15, 16]. This article summarizes the applications of liver organoids and culture systems in recent years and aims to provide insights into effective treatment of liver disease.
Liver organoids derived from liver tissue
The liver's multifunctionality is attributed to its diverse cellular composition and intricate tissue structure. There are two major cell types in the liver: hepatocytes and non-parenchymal cells. Hepatocytes are instrumental in a variety of physiological processes, encompassing exocrine function, gluconeogenesis, detoxification and nutrients storage [17]. In addition to typical liver cells, the parenchymal cells also include intrahepatic bile duct epithelial cells, which are involved in the delivery of intrahepatic bile. While constituting a minor fraction of the liver, non-parenchymal cells exert indispensable roles in many pathophysiologies of the liver. Hepatic stellate cells (HSCs), known to store vitamin A, reside within the perisinusoidal spaces and are activated following liver injury, migrating to affected regions and participating in fibrogenic processes [18]. Hepatic sinusoidal endothelial cells (HSECs), which are predominant in the non-parenchymal cell type, contribute to the formation of sinusoidal walls, thereby optimizing the exchange of substances between hepatocytes and circulatory blood [19]. Kupffer cells (KCs), which are closely associated with the sinusoidal endothelium, develop from monocytes that infiltrate liver tissues. These cells represent a unique subset of liver-resident macrophages [20]. Recent studies, leveraging single-cell techniques, have identified liver progenitor cells in adult liver tissues [21].
The hepatic lobule stands as the fundamental structural and functional unit of the liver. It pivots around the central vein, creating a hexagonal shape. The hepatic artery and portal vein branch inwards from the peripheral regions, ensuring a continuous supply of oxygen and vital nutrients to the liver. Interstitial spaces between hepatocytes give rise to bile canaliculi, which subsequently amalgamate into bile ducts responsible for the efflux of hepatocyte-derived bile [22,23,24]. Given the intricate nature of liver organization, replicating functional lobule configurations ex vivo presents significant challenges. Nevertheless, contemporary strides in organoid methodologies have ushered in opportunities for cultivating and proliferating functional hepatic and bile duct cells in vitro. Liver organoids have been successfully established using isolated adult [25,26,27,28,29] and fetal liver tissues [26, 27, 30], as well as pluripotent stem cells (PSCs) [16, 31,32,33,34,35,36,37,38,39,40,41,42,43]. Under variable culture paradigms, these cells manifest the ability to differentiate into liver organoids bearing specific cellular profiles and architectures. Some researchers have also been done on laboratory animals like mice. Prevailing research avenues are exploring organoids derived from single and composite cell populations. Hepatic primary cells exhibit the potential for organoids formation in both physiological and pathophysiological settings.
Under specific cell culture conditions, primary cells isolated from the liver have the ability to generate organoids. The primary liver parenchymal cells from mice can form organoids in vitro with the presence of WNT signaling pathway agonist, transforming growth factor-β (TGF-β) inhibitor, and the inflammatory cytokine, tumor necrosis factor-α (TNF-α) [44]. In the presence of these factors, mouse liver cells can be passaged in vitro for three months, and biomarkers of hepatic progenitor cells can be detected. In addition to the mouse liver cells, the hepatocytes isolated from human fetuses or adult livers can also form organoids capable of maintaining the liver structure and function for a long time in vitro [45]. Owing to the inherent differences in stemness between adult and fetal liver tissues, the organoids they produce exhibit distinct proliferative behaviors. Organoids derived from adult liver tissues often require a month or more for a single passage, while those originating from fetal liver tissues can be passaged approximately every seven days [28, 46]. Concurrently, there has been research dedicated to inducing differentiation of cholangiocytes extracted from human livers. These endeavors have guided these cells to transition away from their original cholangiocyte identity and toward differentiation into hepatocytes [47]. These liver organoids, characterized by their vesicular structure, manifest select hepatocyte functionalities, notably including albumin secretion and bile production.
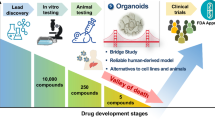
In essence, liver organoids generated from primary human liver tissues have adeptly overcomed the challenges associated with traditional human hepatocytes, which have historically displayed limited in vitro proliferation and passaging potential. This advancement not only enhances the utility of human liver tissues but also presents a groundbreaking model that augments our comprehension of both liver physiology and associated pathological mechanisms. Here, we have also compiled the major applications of liver organoids (Fig. 1 and Table 1), which are elaborated upon in the subsequent sections. However, the present methodology for developing these organoids is not without obstacles. Given the current limitations of establishment of this liver organoids system, such as scarcity of sample sources, suboptimal success rates, and high production costs, this model has yet to completely replace standard 2D culture of human hepatocytes.
Various applications of liver organoids technology. Schematic depiction of liver organoids technology based on existing research. Organoids can be constructed from normal liver, iPSCs, and liver tumor. Such organoids could be used to establish human disease modeling and provide biological resources of organ transplantation. Researchers can also use genetic engineering techniques to study organ development and tumorigenesis in organoids models. Organoids also offer potential tools for drug development and precision medicine. iPSCs, Induced pluripotent stem cells; ALD, Alcohol-related liver disease; NAFLD, Non-alcoholic fatty liver disease
Liver organoids derived from Induced Pluripotent Stem Cells (iPSCs)
Stem cells are a group of cells with vast differentiation potential, forming the foundation for organ regeneration. Human embryonic stem cells (ESCs) derived from the inner cell mass of human blastocysts with the unique capabilities of limitless proliferation, self-renewal, and pluripotency [51]. However, the use of human embryos has consistently faced ethical limits. Takahashi, through gene-editing techniques, introduced transcription factors such as c-MYC into human fibroblasts. Under the culture conditions of ESCs, this resulted in the generation of induced pluripotent stem cells (iPSCs) with stem cell features [52]. The successful establishment of iPSCs sidesteps the ethical dilemmas associated with human embryos. They offer a valuable cellular source for organ transplantation, effectively reducing post-transplant immune rejection, indicating tremendous potential in cell therapy, and are thus widely embraced by the scientific community. Both ESCs and iPSCs can be induced to differentiate into various liver organoid types [10, 53]. This differentiation process simulates the entire developmental journey of the human liver, from embryonic stages to maturation, offering profound insights for research in liver development and regenerative medicine [54, 55]. iPSCs-derived liver organoids are classified as mono-cellular or multi-cellular based on the cells that make them up.
Liver organoids derived from mono-cellular type iPSCs
Liver organoids derived from mono-cellular type iPSCs offer a robust model for exploring hepatocyte structure, function, and mechanisms of hepatocyte damage under pathological conditions. Wang established human ESC-derived expandable hepatic organoids (hEHOs) with bi-directional differentiation potential [31]. Under specific culture conditions, these cells could differentiate into mature hepatocytes or cholangiocytes. These organoids can be successfully passaged in vitro for more than 20 generations. When transplanted into mouse livers, hEHOs not only repaired the damaged tissue but also differentiated into mature hepatocyte-like cells in vivo. Guo differentiated iPSCs into human iPSCs-derived high purity hepatocyte-like cells (iHeps) under standard 2D culture conditions, subsequently cultivating them into iPSCs-derived high purity hepatocyte organoids (iHep-Orgs) [42]. These iHep-Orgs can be passaged in vitro, and compared to iHeps, displayed elevated hepatocyte marker gene expression and increased albumin secretion. However, mono-cellular liver organoids, focusing solely on hepatocytes, lack the diverse liver cell types and the crucial intercellular signaling between hepatocytes and non-hepatocytes, thus falling short of replicating the in vivo hepatic state entirely.
Liver organoids derived from multi-cellular type iPSCs
The successful establishment of multicellular liver organoids is attributed to the pluripotent differentiation characteristics of stem cells and the deepening understanding of liver development. Wu achieved the simultaneous differentiation of endodermal cells and a minority of mesodermal cells by optimizing the culture conditions. Subsequent cultivation resulted in human hepatobiliary organoids (HBOs) [16]. These HBOs contain two essential cell types: hepatocyte-like cells and biliary epithelial-like cells. Hepatocyte-like cells within HBOs demonstrated the ability to synthesize and secrete albumin, alpha-fetoprotein, as well as perform functions such as urea synthesis and glycogen synthesis. When transplanted behind the spleen of immunodeficient mice, HBOs formed biliary-like and hepatocyte-like structures, highlighting their potential therapeutic value in liver transplantation.
Using similar techniques, Bin Ramli and colleagues established HBOs in 96-well plates using 3D suspension culture, with hepatocyte-like cells at the core surrounded by cystic biliary epithelial-like cells [37]. This research not only revealed that each cell type has specific functions but also demonstrated a continuous bile duct system in the organoid that connects hepatocyte-like cell clusters to cystic biliary epithelial-like cells, effectively simulating intrahepatic bile transport. Moreover, these HBOs maintained consistent cellular composition and structural function during large-scale cultivation. This characteristic facilitated the establishment of a drug-induced liver injury model, providing a new platform for high-throughput evaluations of drug-induced hepatotoxicity. In summary, under appropriate induction conditions, iPSCs can differentiate into liver organoids comprising hepatocyte-like cells and a small number of biliary epithelial-like cells within the same system. This model can more accurately predict drug metabolism in the liver and offers a fresh perspective for pre-market evaluation of drug-induced hepatotoxicity.
As previously mentioned, apart from parenchymal cells, the liver also contains non-parenchymal cells like HSCs, HSECs, and KCs, which play roles in maintaining liver function and its pathological alterations [56]. Fatty liver disease (FLD) is a chronic liver condition characterized by excessive fat degeneration, encompassing processes from liver steatosis to inflammation, fibrosis, and cirrhosis. By stimulating HBOs with free fatty acids (FFA), Bin Ramli et al. developed a non-alcoholic fatty liver disease (NAFLD) model that simulates the hepatic lipid degeneration in NAFLD [37]. However, their model only examined lipid changes and did not delve into subsequent occurrence of inflammation and fibrosis. Some researchers dispersed hEHOS into individual cells and then co-cultured them with human fetal liver mesenchymal cells (hFLMCs). After a week of ethanol stimulation, this model displayed lipid accumulation and inflammatory responses [49, 57, 58]. Yet, introducing cells from other sources raises questions about potential immune rejection and whether the model is suitable for personalized drug screening.
Starting with ESCs or iPSCs and differentiating them into liver organoids containing liver-specific cell groups in one system could significantly enhance the precision and stability of liver organoids. Ouchi differentiated ESCs and iPSCs into liver organoids comprising hepatocyte-like cells, stellate-like cells, Kupffer-like cells, and other cell types, each having its respective function [59]. After oleic acid stimulation, they observed lipid accumulation, inflammation, and fibrosis, and they used atomic force microscopy to measure the organoid's stiffness, indicating its fibrosis degree. In subsequent studies, Shinozawa et al. added adding factors like CHIR99021 and A83-01 to increase the efficiency of liver organoid differentiation. Additionally, Shinozawa also utilized liver organoids for high-throughput drug hepatotoxicity testing in vitro and showcased its potential as an in vitro model to predict in idiosyncratic drug hepatotoxicity [59]. Nonetheless, Shinozawa did not explore the interactions between the multiple cell types in their organoids. Based on these studies, Kimura designed a liver organoid biobank from different human sources. They found that some gene mutations prevent fibrosis in non-diabetic conditions but exacerbate non-alcoholic liver disease in diabetic states, underscoring metabolism's role in gene-phenotype associations, and presenting a robust platform for NAFLD precision medicine [43].
The aforementioned studies have successfully demonstrated the feasibility of constructing liver organoids from various cell sources. However, they didn't explore the interactions among the different cell types within these multicellular liver organoids. It's also worth considering whether the spatial distribution and proportion of various cell types in these liver organoids accurately reflect the liver's status within the human body. By adjusting differentiation conditions, there's potential to establish a more stable, controllable, and functionally robust liver organoid model. Liver organoids derived from iPSCs have diversified, and by understanding embryonic organ development, we can adjust cultivation conditions to produce liver organoid models suitable for various research objectives. While iPSCs have addressed the ethical concerns related to ESCs, some studies have reported genetic mutations in iPSCs. In 2015, clinical trials utilizing patient iPSCs were halted due to identified mutations in these cells [60]. A 2022 study reported that iPSCs derived from human skin fibroblasts and blood contain mutations affecting pluripotency. Moreover, those from skin fibroblasts also had UV-induced genetic mutations [61]. This study emphasized cell selection during reprogramming and avoiding prolonged in vitro cultivation to prevent mutation accumulation. Presently, iPSCs play an irreplaceable role in stem cell therapy, necessitating advanced cultivation and quality control techniques to advance their clinical applications. In this context, we have consolidated the cellular composition and fundamental characteristics of liver organoids derived from representative stem cell sources. Additionally, we enumerate the specialized culture systems pertinent to these organoids (Table 2). Detailed methodologies for the cultivation of these organoids are further expounded upon in the subsequent sections.
Liver organoids derived from liver tumors
Primary liver cancer (PLC) is the sixth most common cancer and the fourth leading cause of cancer-related mortality in the world because of late diagnosis and the heterogeneity of the tumor [62,63,64]. Organoids can be used as an excellent cancer model due to the ability of self-renewal and organization, maintenance of the structure and function of the source tissue, and stability of structure and genome in long-term in vitro culture. PLC mainly includes three heterogeneous tumors with different histopathological characteristics: hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma (ICC), and combined hepatocellular-cholangiocarcinoma (cHCC-CCA). Broutier first established primary liver cancer organoids, and conducted research on consistency with primary tumors and drug screening [25]. These organoids can proliferate for a long time and retain the structure, gene expression profiles and genetic characteristics of primary tumors, and provide more accurate research tools for personalized therapy and drug screening. The results showed that liver cancer organoids were suitable for drug screening. Extracellular regulated protein kinases (ERK) inhibitor SCH772984 is a potential therapeutic agent for HCC or cholangiocarcinoma treatment. Similarly, liver cancer organoids are also used to test the drug sensitivity of Sorafinib; it is found that Sorafinib reduces the cell viability of HCC and ICC organoids in a dose-dependent manner [65, 66]. These results indicated that organoids can be used for testing drug specificity and sensitivity. Saito et al. have established differentially gene expression profiles between cholangiocarcinoma and non-cancer organoids; on the other hand, the data of the cholangiocarcinoma cohort in The Cancer Genome Atlas (TCGA) database were analyzed; it was found that COX2 not only showed high expression in cholangiocarcinoma organoids, but also appeared in cholangiocarcinoma cohort in the TCGA database. COX2 should be a promising biomarker for the prognosis of cholangiocarcinoma patients. These finding demonstrated the utility of organoids in molecular marker screening [67]. Other studies established organoid biobanks based on intratumor multiregion sampling, and screened food and drug administration (FDA)-approved antitumor drugs [68]. The results showed that the organoids constructed through multiregion sampling possess heterogeneity in drug sensitivity. In addition, studies also revealed the tumor heterogeneity of organoids by establishing several cases of HCC and ICC organoids [50, 69]. In a genetic manipulation study, after knock-in of the oncogene c-Myc into hepatocyte-derived organoids, the mitochondrial dysfunction and endoplasmic reticulum membrane abnormalities were observed, which may recapitulate the process of cancer development. In the process of inducing the oncogene RAS into organoids, hepatocytes gradually expressed cholangiocyte markers such as CK7. This study found, for the first time, that human hepatocytes can transdifferentiate into ICC, indicating hepatocytes as the cells of origin for ICC development [70]. Cao et al. identified that Sorafenib and Regorafenib exhibited some anti-proliferation effects on mouse tumor organoids [71]. And another study found that Hinokitiol could reduce the proliferation of ICC organoids via inhibiting the ERK signaling pathway activation. Additionally, Hinokitiol combined with Palbociclib (an inhibitor of CDK4) exerts a strong anti-ICC effect [72].
At the same time, the treatment regimens could be dynamically observed and modified using the organoids model; moreover, drug tolerance can also be optimized. In the current research, organoid technology can be taken as the core to establish a living database of biological samples, develop various disease models, and carry out drug-sensitive screening based on 3D biological images. This could greatly contribute to discovering new targets for clinical hepatobiliary diseases, screening and optimizing new drugs, accelerating accurate clinical diagnosis and treatment processes, and shortening the development timeline for innovative drugs (Fig. 1).
Applications of liver organoids
Establishment of disease progression models
Liver organoids can be used as disease models. Alcohol-related liver disease (ALD) is a highly popular chronic liver disease caused by heavy drinking [73]. In a recent study, liver organoids treated with alcohol simulated typical characteristics of the pathophysiology of alcoholic liver injury [31]. Non-alcoholic fatty liver disease (NAFLD) starts with hepatic steatosis, and potentially evolved towards non-alcoholic steatohepatitis (NASH), and even cirrhosis or liver cancer [74]. At present, iPSCs-derived liver organoids reproduce the key features of hepatic steatosis and steatohepatitis in vitro. The continuous stimulation of FFA caused the accumulation of intracellular lipids and increased secretion of inflammatory factors in liver organoids [59]. Considering that NAFLD development involves the interaction of multiple cell types, the liver organoids derived from the co-culture of multiple cell types may be an ideal model for studying NAFLD. Hepatocytes and HSCs play significant roles in liver fibrosis. Co-culturing these two cells in a medium containing fatty acids, palmitic acid, and oleic acid induces lipid accumulation and collagen secretion, while anti-steatotic and anti-fibrotic drugs alleviate these symptoms [75, 76].
One of the principal factors affecting public health is liver cirrhosis and hepatocarcinoma resulting from HBV infection. HBV infection showcases distinct heterogeneity; some individuals exhibit limited or no symptoms, while others progress to cirrhosis or even hepatocarcinoma. Traditional 2D cell culture methods fall short in simulating in vivo conditions, and their limited proliferation capacity hampers viral-related genetic modifications, complicating further research [77]. Organoids possess advantages in long-term preservation, in vitro proliferation, and mimicking cell-tissue interactions, paving the way for disease models of HBV infection that can be preserved in vitro. Overexpression of sodium taurocholate cotransporting polypeptide (NTCP) in liver organoid models tends to facilitate HBV infection, suggesting that NTCP might be one of the crucial receptors for HBV cell invasion [78]. Several researchers have established HCV infection models using hepatoma cell lines. Relative to normal cell lines, defects in the innate immune signaling pathways in hepatoma cell lines present certain challenges to studying the mechanisms of viral infection [77]. iPSCs, with their robust proliferation and differentiation potential, serve as promising cellular models for virus-related studies. Soon Seng Ng utilized polyethylene glycol scaffolds to cultivate iPSCs into organoids, which express genes relevant to viral invasion and packaging, further establishing a post-viral infection organoid model [79]. Nie YZ cultivated liver organoids using iPSCs, bone marrow stromal cells, and HUVECs, subsequently achieving a successful HBV infection [80]. The HBV organoids derived from iPSCs bridge the gaps of traditional models, possessing comprehensive immune-related signaling pathways. Studies on this model hope to pave the way for therapeutic drug development targeting these signaling pathways.
iPSC-derived liver organoids can simulate some liver disease such as Alagille syndrome (ALGS) and cystic fibrosis (CF) [81]. ALGS, an autosomal dominant multisystem disorder associated with various gene mutations, primarily manifests as liver damage due to bile duct abnormalities. CF results from numerous genetic mutations, leading to small bile ducts obstruction within the liver. Lorent observed that treating rodent bile duct cell organoids with bioliatresone resulted in narrowed lumen and disrupted cell structures, confirming that bioliatresone can cause bile duct atresia [81]. These organoids can also be employed to construct congenital liver disease models of bile duct atresia. iPSCs, when differentiated into bile duct cells, form bile duct organoids exhibiting in vivo-like bile duct functionality and markers. Liver organoids cultivated from primary human hepatocytes closely resemble the pathological state of in vivo livers [82]. For instance, in the case of alpha-l antitrypsin deficiency (AATD), a hereditary disease characterized by abnormal ALAT protein folding, studies have shown that liver organoids derived from AATD patient primary hepatocytes can simulate the pathological state of in vivo livers, showcasing ALAT protein aggregation, endoplasmic reticulum stress, and increased cellular apoptosis [83, 84].
Biobank and drug screening
Primary liver cancer stands as one of the leading causes of cancer-related mortality. With increasing living standards, the prevalence of risk factors such as diabetes and obesity has risen, leading to a consequent rise in liver cancer incidence. Whole-genome sequencing has identified a multitude of gene mutations in liver cancer patients, significantly aiding our understanding of the disease. However, the precise mechanisms underpinning liver cancer development remain elusive. Traditional 2D cell cultures fall short in replicating intercellular communication and cell-cell interactions and surrounding cellular environment. 3D cultured tumor cells can more accurately depict the construction of the tumor microenvironment, providing insights for therapeutic targets and drug screening. Employing tumor organoids, as described above, researchers can discern the sensitivities of tumors to various treatments, paving the way for personalized therapies. Building on this foundation, biobanks are established, serving as screening platforms to gauge the efficacy of drugs for liver diseases. Utilizing liver organoids for drug screening signifies a progressive stride in the pharmaceutical sector. Furthermore, biobanks constructed from healthy hepatocytes can serve as platforms to predict drug-induced liver injuries. Mun et.al reported a platform assessing drugs potentially leading to liver toxicity and steatosis [15, 83]. In addition, Shinozawa et al. developed an organoid-based method for drug toxicity evaluation, capable of appraising the hepatic injury toxicity of various marketed drugs, demonstrating remarkable predictive value [38]. A recent study established a liver cancer organoid biobank (LICOB) representing a comprehensive histological and molecular characterization of various liver cancer types, including genomic, epigenomic, transcriptomic, and proteomic data [85]. This study also offers a user-friendly portal, furnishing abundant resources for liver cancer biology and pharmacological dependency research, potentially fostering precision medicine advancements. Concurrently, initiatives are underway to develop a globally accessible organoid culture library, aiming to provide open-source data sharing [86].
Organ development and transplantation
Liver organoids derived from iPSCs serve as effective models for studying liver development. Hepatocyte organoids, cholangiocyte organoids, and hepatocholangiocyte organoids differentiated from iPSCs undergo sequential differentiation, partially recapitulating in vivo liver development [48, 87]. Taking advantage of liver organoid models, studies have revealed that paracrine signals from mesenchymal or endothelial cells promote organoid maturation, mimicking the interactions between matrix cells and epithelial cells during liver development. Current applications of liver organoid transplantation in mouse models have shown therapeutic promise. For instance, Takebe found that after transplanting iPSC-derived organoids into mice with acute liver injury, the organoids rapidly occupied the mouse liver, leading to increased survival time and rate [39]. Additionally, after transplanting liver organoids into a tyrosinemia mouse model, rapid organoid proliferation and significant restoration were observed at the damaged liver sites [44]. Other research indicates that cholangiocyte organoids, when transplanted onto various biocompatible scaffolds, can emulate the bioengineered tissue, successfully repairing the damaged gallbladder wall and extrahepatic ducts in mice [88]. Moreover, studies converting human stem cells into liver organoids using CRISPR technology and subsequently transplanting them into mice with liver injuries have shown detectable human albumin in mouse blood [89].
Acute liver failure mandates liver transplantation as the only effective treatment currently, but the scarcity of liver donors limits its clinical utility. Given that liver organoids can be sustained in an undifferentiated state during in vitro cultivation, they present significant potential as an alternative to organ transplantation therapies. At present, liver transplantation is the effective treatment of acute liver failure, but the shortage of liver source for transplantation limits its clinical application. In the preclinical experiment, the pig liver failure model was established and then treated with pig liver organoids; after treatment, it was observed that the model pig showed a survival time of more than 90 h and a noticeably reduced accumulation level of metabolic wastes relative to the control group [90]. Similar results were also reported in another study of rhesus monkeys. After treatment with the artificial liver technology based on pig liver organoids, the survival rate of lipopolysaccharide (LPS)-induced rhesus monkeys with acute liver failure is effectively improved, accompanied by the weak immune rejection and remarkably improved liver injury status [91]. Because liver organoids can maintain stemness during in vitro culture, they may have large application potential as an organ replacement therapy (Fig. 1) [92].
Common culture medium for liver cancer organoids
Organoids can be differentiated and developed either from adult stem cells (ASCs) or iPSCs through 3D culture. In the process of culturing, the medium conditions should be constantly changed according to the experimental requirements, and different cytokines should be added to induce cell proliferation or directional differentiation. In the early stage of organoids construction, it is necessary to induce cell proliferation and obtain enough cells for the next experiment. Epidermal growth Factor (EGF), fibroblast Growth Factors (FGF), and hepatocyte growth factor (HGF) were added to the cell culture medium. WNT signaling pathway-related regulators such as WNT-3A and R-Spondin 1 (RSPO-1) can effectively promote cell proliferation. The addition of these cytokines allows the cells to expand in vitro for several months, and to express the same markers as the tissues from which they were derived. The above culture methods are suitable for the research needs of most liver organoids. In iPSCs-derived organoids, directional differentiation of cells is often induced according to experimental requirements. For example, after adding bone morphogenetic protein (BMP) and dexamethasone to the culture medium, an increase in the expression of hepatocyte-related markers such as albumin (ALB) was observed, suggesting that the addition of inducible factors promoted the transformation of cells into hepatocytes [31, 47]. The proliferative cells could also be induced for differentiation into cholangiocytes by adding FGF and EGF and adopting 3D matrigel-embedded culture method [15, 16].
The methods for culturing liver tissues and iPSC-derived organoids are intricate. Different culturing systems are constructed based on experimental objectives. In this context, we have consolidated key components used in the induction and cultivation processes of liver organoids from representative stem cell sources (Table 2). At present, with the relatively mature construction methods of liver tumor organoids and medium components, it is easier for researchers to do research [85]. Here, we reviewed the main components of a variety of liver tumor organoids culture media, to provide some theoretical basis for tumor organoids culture (Table 3).
Basic medium
Basic medium is the fundamental components of organoids culture, varying from different tissues. Advanced DMEM/F12 is a commonly used organoid basic medium, which include glucose, non-essential amino acids, and sodium salt. When mammalian cells were cultured with this medium after decreasing the serum content, the growth rate and morphology of the cells did not change [47, 67]. N2 and B27 are commonly used medium supplement that can replace serum. The lack of B27 in neurons will inhibit the phosphorylation of the AKT, which will affect the activity of neurons and inhibit the proliferation, migration and plasticity of neurons [93]. Therefore, it is an essential substance for the culture of organoids. At the same time, N-2-hydroxyethylpiperazine-N-ethane-sulphonicacid (HEPES) buffer was often added to the medium to maintain pH stability.
Amino acids
Amino acids are one of the most basic nutrients organoids culture required. Due to their short half-lives, some amino acids need to be added in freshly prepared medium. The most common three amino acids are L-glutamine, N-acetylcysteine, and nicotinamide. L-glutamine is a common non-essential amino acid in cell culture that, due to its poor stability in solution, can provide an adequate nitrogen source for cells at high concentration in vitro. In general, the L-glutamine concentration in mammalian cell culture medium ranges from 0.68 mmol/L to 4 mmol/L, while that in human peripheral blood is 500 µmol/L. L-glutamine is not only involved in the biosynthesis of cells, but also in the energy metabolism of cells, which is an important part of cell culture [94]. GlutaMAX™, which is chemically more stable, is often used as a substitute for L-glutamine. The most common concentration of GlutaMAX™ is 2 mmol/L. N-acetylcysteine, a precursor of glutathione, is an effective antioxidant and free radical scavenger, and can activate the PI3K/AKT signaling pathway to regulate cell proliferation, differentiation and apoptosis [95]. With the presence of glutathione, N-acetylcysteine would generally not be added to the common culture medium of mammalian cell lines. Nevertheless, the addition of N-acetylcysteine to the organoids culture medium can scavenge the excessive free radicals to the maximum and exert antioxidative effects, thereby promoting cell growth. Nicotinamide, a member of the B vitamin family, is involved in cellular metabolism, oxidation, mitochondrial function, and energy production. Nicotinamide plays an important role in the self-renewal of liver cancer stem cells. Some studies mentioned that nicotinamide can inhibit cell differentiation and promote the amplification of organoids [96]. Similarly, nicotinamide concentration in mammalian cell culture medium ranges from 8 µmol/L to 30 µmol/L, while that in human peripheral blood is 200 µmol/L. However, the commonly used concentration of niacinamide in organoids medium is 10 mmol/L. Nicotinamide with a higher concentration could continuously promote the growth of cells and inhibit cell apoptosis.
Cytokines
Cytokines, a kind of small molecular proteins, are synthesized and secreted by immune cells and non-immune cells. Cytokines can regulate cell growth and differentiation via binding to the corresponding receptors. Interleukin (IL), interferon (IFN), tumor necrosis factor (TNF), chemokine family, and growth factor (GF) are key cytokines. The GF family is particularly important in organoids culture. Since continuous activation of the WNT signaling pathway also plays a crucial role in the process of organoids construction, the WNT pathway activation-related cytokines are often added to the culture medium of organoids. The WNT molecular family consists of 19 members and plays an important role in embryonic development, stem cell differentiation and tumorigenesis. Wnt-3a, one of the family members, is considered to be an activator of WNT/β-catenin signaling pathway and plays a key role in cell proliferation, differentiation and apoptosis [63]. WNT signaling pathway has been confirmed to play a role in the growth and proliferation of a variety of stem cells. It is generally believed that Wnt-3a can bind to the corresponding receptors to form complexes, thereby initiating the transcription of downstream target genes of the WNT signaling pathway to promote the growth and proliferation of stem cell [97]. As an agonist, RSPO-1 can also activate the WNT signaling pathway. It has been proposed that RSPO-1 can maintain the continuous activation of WNT signaling pathway after binding to leucine-rich repeat-containing G protein-coupled receptor 5 (LGR5), thereby promoting the proliferation and division of stem cells and maintaining the phenotype of stem cells [98, 99]. As an activator of WNT signaling pathway, RSPO-1 plays a supporting role in the development and growth of liver organoids [100]. Noggin is a secreted protein that controls the self-renewal of stem cells by binding to BMP4 and BMP7. It has been confirmed that the expression of the stem cell marker LGR5 is decreased in mouse liver organoids under Noggin deficient conditions [101]. Therefore, Noggin may exert its effect on LGR5 expression, and can be used for the long-term culture of liver organoids [25]. EGF is an important cytokine in human body, and its function is mainly to promote cell proliferation. EGF signaling pathway also plays an important role in organoids growth and promotes the proliferation of cancer stem cells [102]. FGF is widely distributed in a variety of tissues and organs in the body that regulates cell growth, proliferation, and differentiation. The most common family members in tumor organoids culture are FGF2, FGF7, and FGF10. FGF10 can promote the morphogenesis of organoids and maintain the stemness of embryonic stem cells, so it has become one of the most common cytokines in liver tumor organoids culture in vitro [77, 103]. HGF can promote the proliferation of hepatocytes and has been widely used in organoids culture. A study found that combined HGF and RSPO-1 could promote the expression of endogenous LGR5+ cells, thereby promoting the formation of liver organoids [104].
Small molecule compounds
Y-27632 is an inhibitor of Rho-associated coiled-coil containing protein kinase (ROCK). The ROCK signaling pathway plays an important role in the regulation of cell growth, proliferation, and apoptosis [105]. Y-27632 can promote stem cell self-renewal and proliferation. Currently, Y-27632 has been used as a culture adjuvant in a variety of organoids to prevent cell apoptosis and promote organoids formation [106, 107]. TGF-β signaling pathway also plays a key role in regulating cell proliferation and apoptosis [108, 109]. As an inhibitor of TGF-β receptor, A83-01 can promote organoids formation [110]. The combination of Y-27632 and A83-01 increased the cell viability and proliferation ability [111]. In addition, forskolin, as an activator of adenylate cyclase, has been widely used in liver organoids cultures [32].
Scaffolds for organoids culture
During the cultivation process of organoids, the scaffolds of cell culture can provide a microstructure that is suitable for cell growth. The ideal scaffolds can promote cell growth and adhesion, including the nutritional environment that maintains cell vitality, and the biodegradation rate is similar to the substrate deposition rate of cells in the new environment [79, 112]. Matrigel is a gelatinous protein mixture secreted by Engelbreth-Holm-Sarm (EHS) mouse sarcoma cells that provides structural support and extracellular signaling to cells [113]. However, the complex and variable composition also makes it difficult to control the culture environment and reduces repeatability. At present, decellularized scaffolds and hydrogels have been used in organoids culture systems [114, 115]. Acellular scaffold refers to the use of enzymes to remove cells from isolated tissues and organs while retaining the basic structure and extracellular matrix of organs. The simulated culture environment cannot reproduce the dynamic changes of cell living environment at present. Decellularized scaffolds have natural advantages in this regard, such as large-scale retention of various components in the cell growth environment, and meeting the necessary support structure and material delivery for organoids growth [116]. Currently, hydrogels are also a direction for organoids culture. Hydrogels can be obtained from materials such as collagen, fibrin, and chitosan, which have high biocompatibility and low toxicity. While performing a supporting role, cytokines and nutrients can also be delivered through the hydrogel [112]. With the development of materials science and 3D printing technology, new cell culture scaffolds are constantly emerging. Materials such as polylactic acid and polyethylene glycol have been applied to the synthesis of cell culture scaffolds [79]. Artificial cell culture scaffolds lack the conditions to promote cell adhesion and growth, and often need to be coated with extracellular matrix proteins on the surface of the scaffold. Collagen I can better promote cell adhesion and proliferation on scaffolds and is a common surface coating material in cell scaffolds [117]. In addition to the above-mentioned cell scaffolds, Organoids-on-Chips, and culturing organoids directly in suspension also provide new ideas for organoids culture [118,119,120]. The innovative cultivation methods such as other co-culturing systems, organoid chips, and direct suspension-based organoid culturing offer fresh perspectives for organoid cultivation. With the advancement of precision manufacturing technologies and microfluidic techniques, it has become possible to simulate the in vivo microenvironments externally using specialized materials and methodologies [121]. Simoneau et al. co-cultured organoids derived from human liver progenitor cells and CD8+ T cells from hepatitis C patients within a microfluidic system. This setup emulates the T cell invasion and physiological changes in the liver during hepatitis C infection, assisting in the development of vaccines against the hepatitis C virus [122]. However, the initial cultivation of the liver organoids in this model still relied on the support of Matrigel. The presence and amount of Matrigel used in the co-culturing process within the microfluidic system cannot be overlooked given its potential impact on the experiments.
Opportunities and challenges
Organoids is a new model that recapitulates the genetic characteristics and heterogeneity of tissues, showing a promising prospect. In addition, organoids, as a special biological sample, can be frozen and resuscitated, which can help researchers understand, diagnose, and prevent refractory diseases such as developmental disorders, tumor, and genetic diseases. In the field of liver research, organoids can be used to simulate liver development and tumor formation in vitro, and to construct various disease models such as ALD and NAFLD. Especially for cancer patients, multidisciplinary comprehensive treatment mode will become the general trend, and each patient will obtain individualized treatment plan through in vitro experiments of tumor organoids derived from the patient. Organoids in vitro can also replace the need for organ donation and provide convenience for patients in urgent need of organ transplantation.
Currently, organoids can be successfully constructed from various tissue sources. Ongoing research calls for the development of more complex organoid culture systems and the expansion of co-culture systems involving various organoids with other biological tissues. Here, we summarize some potential shortcomings in organoid applications (Fig. 2). With technological advancements, we believe these issues can be effectively addressed.
A schematic overview of future development in the field of liver organoids. Schematic diagram illustrating the future development directions of liver organoids. It is crucial to standardize the methods for constructing, culturing, and preserving liver organoids. Future research will require more advanced analytical and detection techniques to assess and confirm the consistency between liver organoids and their source tissues. Additionally, there should be a greater focus on enhancing the vascularization, systematization, and co-culture systems of liver organoids
The technological schools of liver organoids construction
Liver tissue, liver cancer tissue, ESCs, and iPSCs can all serve as sources for liver organoids. In the field of cell transdifferentiation, Hui used self-developed culture media and differentiation techniques to convert fibroblast cells into liver organoids, successfully constructing liver organoids with polygonal structures [70, 123]. Liver organoids derived from ESCs/iPSCs are not limited by liver tissue availability. Although PSCs have the potential to differentiate into all cell types of the three germ layers, the typical differentiation process involves first determining the direction of differentiation of a specific germ layer, followed by gradual differentiation into corresponding progenitor cells and mature cell types under the regulation of various signaling factors. Consequently, the organoids generated from ESC differentiation usually consist of epithelial cell types but lack supportive cell types such as stromal and mesenchymal cells. Liver organoids derived from iPSCs by Mun et al. only contain a single epithelial cell type [124]. These organoids can differentiate into mature liver cells under the influence of differentiation culture media. In contrast, liver organoids developed by the Guo simultaneously differentiate into liver organoids containing both epithelial and stromal cell lineages. Organoids obtained through this method can differentiate into multiple cell types, with hepatocytes accounting for 59.2%, bile duct cells 9%, stellate cells 31%, and immune Kupffer cells 0.8%. Liver organoids derived from ESCs often exhibit phenotypes reminiscent of embryonic liver, indicating immature characteristics [42]. There are significant differences in function and gene expression between embryonic and adult livers. Mun et al. stimulated the maturation of liver organoids using microbial metabolites such as short-chain fatty acids, which increased albumin secretion and gene expression in liver cells [124]. However, it is worth noting that genetic and epigenetic abnormalities, such as copy number variations (CNVs) and point mutations in protein-coding regions, are often encountered during iPSC reprogramming and organoid differentiation [60, 61]. In contrast, the representative work of constructing organoids from adult stem cells (ASCs) was carried out by the Hans clevers. Their organoids, cultivated through three months of continuous passaging, exhibited only one-tenth of the genetic mutations observed in liver organoids derived from iPSCs [28]. In addition, a recent study employed single-cell sequencing technology to analyze organoids derived from liver tumors, investigating the tumors' heterogeneity and evolutionary patterns. These findings further substantiate the capability of organoids to faithfully mirror the heterogeneity observed in tumors [50].
Therefore, the most important and crucial criteria for evaluating liver organoids lie in their structural and functional similarity to primary liver tissue. Evaluation can be conducted through three main aspects: a) single-cell sequencing and gene expression profiling to analyze the similarity with source tissues; b) immunohistochemical staining, flow cytometry, and other techniques to analyze cell phenotypes; c) functional assays, such as measuring albumin secretion, to assess hepatocyte function. Considering the status of these three technical approaches, organoids derived from iPSCs are prone to epigenetic abnormalities and can be considered hepatocyte-like organoids. Liver organoids derived from ESCs exhibit characteristics of embryonic liver, while the functional and gene expression differences between embryonic and adult livers remain significant. Achieving a mature state in these organoids is critical for disease modeling and drug testing. Liver organoids derived from ASCs are directly derived from primary tissues and therefore closely mimic physiological states. The current landscape sees a flourishing development of technologies, including single-cell analysis. Nevertheless, these methodologies are seldom employed to evaluate the similarity between liver organoids and their source tissues, or to compare the cellular composition differences among organoids from varying sources. Future research in this realm holds significant potential for exploration.
Vascularization of liver organoids
Co-culturing organoids with endothelial cells (ECs) enhances organoid vascularization, mimicking the natural functionality of solid organs and providing a closely representative internal environment for basic and biomedical research. Vascularized brain organoids offer an effective platform for modeling neurovascular diseases. Salmon et al. utilized custom 3D-printed microfluidic chips to organize periosteal cells derived from pluripotent stem cells (PSCs) and ECs into vascular networks, creating fully formed neurovascular organoids [125]. In HCC research, anti-angiogenic therapy has emerged as a standard treatment. Recently, researchers employed a hydrogel system to mimic and characterize the interaction between HCC and endothelial cells in vitro. Co-culturing xenograft organoids from patient-derived organoids with endothelial cells led to an upregulation of interleukin-8 (IL-8), indicating that the tumor cell-endothelial interaction in the co-culture model reproduced signals for tumor-induced vascular secretion [126]. In this model, the interaction between HCC and endothelial cells induced macrophage polarization towards a pro-inflammatory and pro-angiogenic phenotype, aligning with previously reported tumor-associated macrophage phenotypes. However, a current limitation is that our tumor-vascular system inadequately reproduces the complex process of tumor-induced angiogenesis. Furthermore, the tumor microenvironment lacks certain components, as it includes not only tumor cells and vascular endothelial cells, but also other cell types and structural components that existing systems cannot fully replicated. Similarly, the liver consists of various cell types, and not all are currently incorporated into liver organoid models. Developing a model that includes all cell types in their native ratios will be crucial to ensure the organoid's functionality fully mimics the liver. Another limitation of organoid culture is the lack of blood vessels that facilitate nutrient exchange. The development of an intricate blood vessel network is crucial for nutrient delivery and waste removal in these models. Actually, we must construct organoids with sufficient volume to simulate the complex structure of the system. Such models are essential for predicting the effectiveness of targeted therapies, as demonstrated by anti-angiogenic therapy for tumors.
Systematization of liver organoids
Numerous diseases arise from the coordinated interaction of multiple organ systems. While traditional single-organoid models can accurately replicate the environment of individual organs in the body, they still exhibit limitations in addressing diseases involving multiple systems. Consequently, the development of multi-organoid models capable of simulating the coordinated interactions of various systems has emerged as a research direction to tackle this challenge. Increasing research suggests that the maintenance and pathology of liver functions are closely linked with other organs like the intestine, gallbladder, and pancreas [127]. This implies the importance of considering inter-organ interactions when establishing liver organoid models. In earlier researches, Takebe co-cultured hepatic cells differentiated from hiPSCs, HUVECs, and mesenchymal stem cells (MSCs), and spontaneously formed liver bud structures with vascular-like networks, exhibiting some functions of hepatocytes [39]. When these liver buds were transplanted into mice, they contributed to vascular formation and had therapeutic effects on liver-damaged mice [40]. Koike further achieved co-cultivation of early embryonic foregut and hindgut spheroids differentiated from iPSCs, resulting in the formation of hepatobiliary-pancreatic organoids (HBPOs) [34]. This model serves as a robust platform for studying the interactions among the liver, gallbladder, and pancreas during their developmental phases.
Both models underscore the influence of blood flow and inter-organ interactions on normal liver physiology. Externally, it's feasible to incorporate these elements into liver organoid models using microfluidic technologies. Wang et al. developed a liver organoid-on-a-chip using polydimethylsiloxane [33]. This design accommodates 3D cell culturing while maintaining media circulation. Compared to traditional methods, this approach replaces the Matrigel with biosynthetic inorganic materials, eliminating the effects of heterologous organic components on the organoids. Wang found that organoids differentiated from iPSCs under media flow conditions demonstrated higher albumin secretion and cell vitality than their statically-cultured counterparts. Introducing FFA to the culture medium highlighted evident lipid droplet accumulation within the liver organoids. Furthermore, inflammatory and fibrosis markers were upregulated in the FFA group [128]. Tao et al. expanded on this research by modifying the organoid chip to co-culture two types of organoids within a single circulatory system, creating an iPSC-derived liver-islet organoid model [53]. When cultured under the same conditions, co-cultured organoids showcased superior cell vitality and organ function maturity compared to their individually-cultured counterparts, further emphasizing the significance of inter-organ interactions. Furthermore, the failure of current organoids cultures to reproduce the complex networks between different systems of the body is a limiting factor in studying the coordinated role of different organs.
Co-culture system of liver organoids
Human immune cells play a crucial role in liver formation and the progression of liver tumors. Liver tumor organoids are of great significance in the precise treatment of patients. In tumor research, the tumor immune microenvironment is essential. In order to better preserve tumor heterogeneity and tumor immune microenvironment, a variety of organoids culture systems have been established. From the basic organoids embedding method, the air-liquid interface culture system and co-culture system were derived. In recent years, several studies have successfully constructed tumor immune organoids systems by Holistic or Reductionist methods [129,130,131]. Neal et al. constructed a model of organoids through the Holistic culture system, which contains both epithelium cells and stroma cells, as well as specific tumor-infiltrating lymphocytes (TILs) [131]. In addition to the basic medium, this culture system was supplemented with additional T cell activators, such as interleukin-2 (IL-2), to support the growth of immune cells. Therefore, many types of immune cells, such as CD8+ T cells, CD4+ T cells, B cells, natural killer (NK) cells and natural killer T (NKT) cells, can growth in this system. In the reductionist culture system, organoids can be co-cultured with immune cells from the peripheral blood of the same patient [130]. Currently, various immunotherapeutic approaches hold promise for fundamentally changing the treatment of cancer patients. Programmed death-1 (PD-1) and programmed death ligand-1 (PD-L1) blockade are the most effective immunotherapy targeting T cell inhibitory checkpoint receptors, yet their response rate in most cancer types is only 15% to 25% [132]. Therefore, establishing reliable in vitro models to assess efficacy and optimize immunotherapy methods is essential. Chakrabarti et al. used autologous human gastric cancer organoids co-cultured with patient immune cells as a preclinical research model to predict the effectiveness of PD-L1-targeted therapy, aiming to improve efficacy [133]. In the field of hepatobiliary tumors, Zhou co-cultured bile duct cancer organoids with peripheral blood mononuclear cells or T cells, showing an effective antitumor T cell immune response in the co-culture, including organoid killing, increased cell apoptosis, and release of soluble factors [134]. This system can predict which immune therapy is most effective for individual patients, which may be an important step in personalized immunotherapy for CCA. In terms of liver metastases, Liu transplanted prostate cancer organoids into the prostate of C57BL/6 mice and then established a prostate cancer liver metastasis model with an immune microenvironment, which can serve as a valuable in vivo validation model for immunotherapy [135].
In addition to T cells, other cells in the immune microenvironment are also particularly important. Cancer-associated fibroblasts (CAFs) are an important cellular component in the tumor microenvironment [136]. Öhlund et al. co-cultured pancreatic ductal adenocarcinoma organoids with mouse pancreatic stellate cells and found that stellate cells transformed into different subtypes of CAFs in different ways, indicating the heterogeneity of CAFs in the tumor [137]. With the successful construction of various organoids, exploration of co-culture systems is also rapidly progressing. However, there are still uncertainties about how to select culture media and matrices for co-culture methods. Therefore, to study the interactions between organoids and immune cells, nerve cells, fibroblasts, etc., co-culture methods must be employed. However, there are still uncertainties about how to select culture media for co-culture methods, and whether the results obtained under compromise culture conditions can represent real responses under physiological conditions remains unclear.
The common components of organoids culture medium have been well recognized, but the antagonistic effects and dosage of some specific culture components still need to be explored. At the same time, the role of different culture components in different organoids models still needs to be further clarified. For the development of organoids for clinical application, the components ratio of culture medium and the operation in the culture process should be standardized. Constructing a liver organoids model is intricate, with numerous variables affecting its success, including the matrix's composition, cell ratio, and the culture medium. Organoid models must be standardized to ensure their consistent application in research.
Standardization of the construction, culture, and maintenance of liver organoids
Considering ongoing investigations in liver organoid research, we propose that the following considerations be taken into account when building organoid sample libraries to enhance success rates and scientific reproducibility [85]. (a) After detaching tissue, it is advisable to pre-wash it with a sterile PBS solution or physiological saline to remove bloodstains and contaminants, as obtaining highly active samples is crucial for successful organoid construction. (b) For different tissues, determining the appropriate sample digestion time is important to avoid over-digestion. (c) Using a low-growth factor matrix can prevent organoids from being influenced by cytokines, which could lead to undirected differentiation or apoptosis. (d) It is important to maintain a low temperature and work quickly during operations involving the matrix. (e) Signs such as uneven boundaries, darkening color of organoids indicate trends like apoptosis or differentiation in organoids, necessitating immediate passaging. Additionally, when the matrix structure starts to loosen, prompt passaging is required to ensure organoids have a complete culture environment and can evenly absorb nutrients. Generally, organoids should be passaged when their diameter reaches 200 μm. f) Organoids should be cryopreserved in a well-growing state, with a recommended cryopreservation volume in each tube above 2 × 105/mL. The diameter of the organoids should be less than 100 μm for cryopreservation. This ensures the cryoprotectant penetrates effectively.
As research progresses, the refinement and standardization of these models will undoubtedly pave the way for even more breakthroughs in hepatology. In the future, with technological innovation, liver organoids will be more widely applied and clinically transformed in the fields of drug development, disease modeling and personalized treatment.
Availability of data and materials
Not applicable.
References
Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6.
Ju HL, Han KH, Lee JD, et al. Transgenic mouse models generated by hydrodynamic transfection for genetic studies of liver cancer and preclinical testing of anti-cancer therapy. Int J Cancer. 2016;138(7):1601–8.
Nakayama J, Gong Z. Transgenic zebrafish for modeling hepatocellular carcinoma. MedComm (2020). 2020;1(2):140–56.
Kostadinova R, Boess F, Applegate D, et al. A long-term three dimensional liver co-culture system for improved prediction of clinically relevant drug-induced hepatotoxicity. Toxicol Appl Pharmacol. 2013;268(1):1–16.
Meli L, Jordan ET, Clark DS, et al. Influence of a three-dimensional, microarray environment on human cell culture in drug screening systems. Biomaterials. 2012;33(35):9087–96.
Duval K, Grover H, Han LH, et al. Modeling physiological events in 2D vs. 3D cell culture. Physiology (Bethesda). 2017;32(4):266–77.
Rowe RG, Daley GQ. Induced pluripotent stem cells in disease modelling and drug discovery. Nat Rev Genet. 2019;20(7):377–88.
Mariotti V, Strazzabosco M, Fabris L, et al. Animal models of biliary injury and altered bile acid metabolism. Biochim Biophys Acta Mol Basis Dis. 2018;1864(4 Pt B):1254–61.
Fatehullah A, Tan SH, Barker N. Organoids as an in vitro model of human development and disease. Nat Cell Biol. 2016;18(3):246–54.
Akbari S, Sevinc GG, Ersoy N, et al. Robust, long-term culture of endoderm-derived hepatic organoids for disease modeling. Stem Cell Rep. 2019;13(4):627–41.
McCracken KW, Cata EM, Crawford CM, et al. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature. 2014;516(7531):400–4.
Pang MJ, Burclaff JR, Jin R, et al. Gastric organoids: progress and remaining challenges. Cell Mol Gastroenterol Hepatol. 2022;13(1):19–33.
Hattori N. Cerebral organoids model human brain development and microcephaly. Mov Disord. 2014;29(2):185.
Qian X, Song H, Ming GL. Brain organoids: advances, applications and challenges. Development. 2019;146(8):dev166074.
Prior N, Inacio P, Huch M. Liver organoids: from basic research to therapeutic applications. Gut. 2019;68(12):2228–37.
Wu F, Wu D, Ren Y, et al. Generation of hepatobiliary organoids from human induced pluripotent stem cells. J Hepatol. 2019;70(6):1145–58.
Morigny P, Boucher J, Arner P, et al. Lipid and glucose metabolism in white adipocytes: pathways, dysfunction and therapeutics. Nat Rev Endocrinol. 2021;17(5):276–95.
Kisseleva T, Brenner D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol. 2021;18(3):151–66.
Gracia-Sancho J, Caparros E, Fernandez-Iglesias A, et al. Role of liver sinusoidal endothelial cells in liver diseases. Nat Rev Gastroenterol Hepatol. 2021;18(6):411–31.
Li W, Chang N, Li L. Heterogeneity and function of kupffer cells in liver injury. Front Immunol. 2022;13:940867.
Aizarani N, Saviano A, Sagar N, et al. A human liver cell atlas reveals heterogeneity and epithelial progenitors. Nature. 2019;572(7768):199–204.
Ben-Moshe S, Itzkovitz S. Spatial heterogeneity in the mammalian liver. Nat Rev Gastroenterol Hepatol. 2019;16(7):395–410.
Manco R, Itzkovitz S. Liver zonation. J Hepatol. 2021;74(2):466–8.
Paris J, Henderson NC. Liver zonation, revisited. Hepatology. 2022;76(4):1219–30.
Broutier L, Mastrogiovanni G, Verstegen MM, et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat Med. 2017;23(12):1424–35.
Hu H, Gehart H, Artegiani B, et al. Long-term expansion of functional mouse and human hepatocytes as 3D organoids. Cell. 2018;175(6):1591–606.
Huch M, Dorrell C, Boj SF, et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013;494(7436):247–50.
Huch M, Gehart H, van Boxtel R, et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. 2015;160(1–2):299–312.
Zhang RR, Koido M, Tadokoro T, et al. Human iPSC-derived posterior gut progenitors are expandable and capable of forming gut and liver organoids. Stem Cell Rep. 2018;10(3):780–93.
Vyas D, Baptista PM, Brovold M, et al. Self-assembled liver organoids recapitulate hepatobiliary organogenesis in vitro. Hepatology. 2018;67(2):750–61.
Wang S, Wang X, Tan Z, et al. Human ESC-derived expandable hepatic organoids enable therapeutic liver repopulation and pathophysiological modeling of alcoholic liver injury. Cell Res. 2019;29(12):1009–26.
Wang X, Ni C, Jiang N, et al. Generation of liver bipotential organoids with a small-molecule cocktail. J Mol Cell Biol. 2020;12(8):618–29.
Wang Y, Wang H, Deng P, et al. In situ differentiation and generation of functional liver organoids from human iPSCs in a 3D perfusable chip system. Lab Chip. 2018;18(23):3606–16.
Koike H, Iwasawa K, Ouchi R, et al. Modelling human hepato-biliary-pancreatic organogenesis from the foregut-midgut boundary. Nature. 2019;574(7776):112–6.
Ogawa M, Ogawa S, Bear CE, et al. Directed differentiation of cholangiocytes from human pluripotent stem cells. Nat Biotechnol. 2015;33(8):853–61.
Pollen AA, Bhaduri A, Andrews MG, et al. Establishing cerebral organoids as models of human-specific brain evolution. Cell. 2019;176(4):743–56.
Ramli M, Lim YS, Koe CT, et al. Human pluripotent stem cell-derived organoids as models of liver disease. Gastroenterology. 2020;159(4):1471–86.
Shinozawa T, Kimura M, Cai Y, et al. High-fidelity drug-induced liver injury screen using human pluripotent stem cell-derived organoids. Gastroenterology. 2021;160(3):831–46.
Takebe T, Sekine K, Enomura M, et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499(7459):481–4.
Takebe T, Sekine K, Kimura M, et al. Massive and reproducible production of liver buds entirely from human pluripotent stem cells. Cell Rep. 2017;21(10):2661–70.
Collin DLA, Takeishi K, Guzman-Lepe J, et al. Generation of human fatty livers using custom-engineered induced pluripotent stem cells with modifiable SIRT1 metabolism. Cell Metab. 2019;30(2):385–401.
Guo J, Duan L, He X, et al. A combined model of human iPSC-derived liver organoids and hepatocytes reveals ferroptosis in DGUOK mutant mtDNA depletion syndrome. Adv Sci (Weinh). 2021;8(10):2004680.
Kimura M, Iguchi T, Iwasawa K, et al. En masse organoid phenotyping informs metabolic-associated genetic susceptibility to NASH. Cell. 2022;185(22):4216–32.
Peng WC, Logan CY, Fish M, et al. Inflammatory cytokine TNFalpha promotes the long-term expansion of primary hepatocytes in 3D culture. Cell. 2018;175(6):1607–19.
Katsuda T, Kawamata M, Hagiwara K, et al. Conversion of terminally committed hepatocytes to culturable bipotent progenitor cells with regenerative capacity. Cell Stem Cell. 2017;20(1):41–55.
Sato T, Stange DE, Ferrante M, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 2011;141(5):1762–72.
Broutier L, Andersson-Rolf A, Hindley CJ, et al. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat Protoc. 2016;11(9):1724–43.
Camp JG, Sekine K, Gerber T, et al. Multilineage communication regulates human liver bud development from pluripotency. Nature. 2017;546(7659):533–8.
Si-Tayeb K, Lemaigre FP, Duncan SA. Organogenesis and development of the liver. Dev Cell. 2010;18(2):175–89.
Zhao Y, Li ZX, Zhu YJ, et al. Single-cell transcriptome analysis uncovers intratumoral heterogeneity and underlying mechanisms for drug resistance in hepatobiliary tumor organoids. Adv Sci (Weinh). 2021;8(11):e2003897.
Kong M, Zhou D. Establishment of universal human embryonic stem cell lines. Immunol Lett. 2021;230:59–62.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76.
Tao T, Deng P, Wang Y, et al. Microengineered multi-organoid system from hiPSCs to recapitulate human liver-islet axis in normal and type 2 diabetes. Adv Sci (Weinh). 2022;9(5):e2103495.
Jalan-Sakrikar N, Brevini T, Huebert RC, et al. Organoids and regenerative hepatology. Hepatology. 2023;77(1):305–22.
Takebe T, Taniguchi H. Human iPSC-derived miniature organs: a tool for drug studies. Clin Pharmacol Ther. 2014;96(3):310–3.
Bonnardel J, T’Jonck W, Gaublomme D, et al. Stellate cells, hepatocytes, and endothelial cells imprint the kupffer cell identity on monocytes colonizing the liver macrophage niche. Immunity. 2019;51(4):638–54.
Joshi M, B P P, He Z, et al. Fetal liver-derived mesenchymal stromal cells augment engraftment of transplanted hepatocytes. Cytotherapy. 2012;14(6):657–69.
Mederacke I, Hsu CC, Troeger JS, et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun. 2013;4:2823.
Ouchi R, Togo S, Kimura M, et al. Modeling steatohepatitis in humans with pluripotent stem cell-derived organoids. Cell Metab. 2019;30(2):374–84.
Garber K. RIKEN suspends first clinical trial involving induced pluripotent stem cells. Nat Biotechnol. 2015;33(9):890–1.
Rouhani FJ, Zou X, Danecek P, et al. Substantial somatic genomic variation and selection for BCOR mutations in human induced pluripotent stem cells. Nat Genet. 2022;54(9):1406–16.
Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Gao K, Zhang T, Wang F, et al. Therapeutic potential of Wnt-3a in neurological recovery after spinal cord injury. Eur Neurol. 2019;81(3–4):197–204.
Marquardt JU, Andersen JB, Thorgeirsson SS. Functional and genetic deconstruction of the cellular origin in liver cancer. Nat Rev Cancer. 2015;15(11):653–67.
Aberle MR, Burkhart RA, Tiriac H, et al. Patient-derived organoid models help define personalized management of gastrointestinal cancer. Br J Surg. 2018;105(2):e48–60.
Nuciforo S, Fofana I, Matter MS, et al. Organoid models of human liver cancers derived from tumor needle biopsies. Cell Rep. 2018;24(5):1363–76.
Saito Y, Muramatsu T, Kanai Y, et al. Establishment of patient-derived organoids and drug screening for biliary tract carcinoma. Cell Rep. 2019;27(4):1265–76.
Li L, Knutsdottir H, Hui K, et al. Human primary liver cancer organoids reveal intratumor and interpatient drug response heterogeneity. JCI Insight. 2019;4(2):e121490.
Yuan B, Zhao X, Wang X, et al. Patient-derived organoids for personalized gallbladder cancer modelling and drug screening. Clin Transl Med. 2022;12(1):e678.
Sun L, Wang Y, Cen J, et al. Modelling liver cancer initiation with organoids derived from directly reprogrammed human hepatocytes. Nat Cell Biol. 2019;21(8):1015–26.
Cao W, Liu J, Wang L, et al. Modeling liver cancer and therapy responsiveness using organoids derived from primary mouse liver tumors. Carcinogenesis. 2019;40(1):145–54.
Bai P, Ge C, Yang H, et al. Screening a redox library identifies the anti-tumor drug Hinokitiol for treating intrahepatic cholangiocarcinoma. Front Biosci (Landmark Ed). 2022;27(1):18.
Seitz HK, Bataller R, Cortez-Pinto H, et al. Alcoholic liver disease. Nat Rev Dis Primers. 2018;4(1):16.
Friedman SL, Neuschwander-Tetri BA, Rinella M, et al. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24(7):908–22.
Kruitwagen HS, Oosterhoff LA, Vernooij I, et al. Long-term adult feline liver organoid cultures for disease modeling of hepatic steatosis. Stem Cell Rep. 2017;8(4):822–30.
Pingitore P, Sasidharan K, Ekstrand M, et al. Human multilineage 3D spheroids as a model of liver steatosis and fibrosis. Int J Mol Sci. 2019;20(7):1629.
Fujii M, Matano M, Toshimitsu K, et al. Human intestinal organoids maintain self-renewal capacity and cellular diversity in niche-inspired culture condition. Cell Stem Cell. 2018;23(6):787–93.
Baktash Y, Madhav A, Coller KE, et al. Single particle imaging of polarized hepatoma organoids upon hepatitis c virus infection reveals an ordered and sequential entry process. Cell Host Microbe. 2018;23(3):382–94.
Ng SS, Saeb-Parsy K, Blackford S, et al. Human iPS derived progenitors bioengineered into liver organoids using an inverted colloidal crystal poly (ethylene glycol) scaffold. Biomaterials. 2018;182:299–311.
Nie YZ, Zheng YW, Miyakawa K, et al. Recapitulation of hepatitis B virus-host interactions in liver organoids from human induced pluripotent stem cells. EBioMedicine. 2018;35:114–23.
Lorent K, Gong W, Koo KA, et al. Identification of a plant isoflavonoid that causes biliary atresia. Sci Transl Med. 2015;7(286):286ra67.
Guan Y, Xu D, Garfin PM, et al. Human hepatic organoids for the analysis of human genetic diseases. JCI Insight. 2017;2(17):e94954.
Gomez-Mariano G, Matamala N, Martinez S, et al. Liver organoids reproduce alpha-1 antitrypsin deficiency-related liver disease. Hepatol Int. 2020;14(1):127–37.
Zeilinger K, Freyer N, Damm G, et al. Cell sources for in vitro human liver cell culture models. Exp Biol Med (Maywood). 2016;241(15):1684–98.
Ji S, Feng L, Fu Z, et al. Pharmaco-proteogenomic characterization of liver cancer organoids for precision oncology. Sci Transl Med. 2023;15(706):eadg3358.
Drost J, Clevers H. Organoids in cancer research. Nat Rev Cancer. 2018;18(7):407–18.
Asai A, Aihara E, Watson C, et al. Paracrine signals regulate human liver organoid maturation from induced pluripotent stem cells. Development. 2017;144(6):1056–64.
Sampaziotis F, Justin AW, Tysoe OC, et al. Reconstruction of the mouse extrahepatic biliary tree using primary human extrahepatic cholangiocyte organoids. Nat Med. 2017;23(8):954–63.
Velazquez JJ, LeGraw R, Moghadam F, et al. Gene regulatory network analysis and engineering directs development and vascularization of multilineage human liver organoids. Cell Syst. 2021;12(1):41–55.
Chen HS, Joo DJ, Shaheen M, et al. Randomized trial of spheroid reservoir bioartificial liver in porcine model of posthepatectomy liver failure. Hepatology. 2019;69(1):329–42.
Li Y, Wu Q, Wang Y, et al. Novel spheroid reservoir bioartificial liver improves survival of nonhuman primates in a toxin-induced model of acute liver failure. Theranostics. 2018;8(20):5562–74.
Reza HA, Okabe R, Takebe T. Organoid transplant approaches for the liver. Transpl Int. 2021;34(11):2031–45.
Alvarez-Flores MP, Hebert A, Gouelle C, et al. Neuroprotective effect of rLosac on supplement-deprived mouse cultured cortical neurons involves maintenance of monocarboxylate transporter MCT2 protein levels. J Neurochem. 2019;148(1):80–96.
Li J, Ghazwani M, Liu K, et al. Regulation of hepatic stellate cell proliferation and activation by glutamine metabolism. PLoS One. 2017;12(8):e182679.
Hamid ZA, Tan HY, Chow PW, et al. The role of N-Acetylcysteine supplementation on the oxidative stress levels, genotoxicity and lineage commitment potential of ex vivo murine haematopoietic stem/progenitor cells. Sultan Qaboos Univ Med J. 2018;18(2):e130–6.
Guo Q, Chen S, Rao X, et al. Inhibition of SIRT1 promotes taste bud stem cell survival and mitigates radiation-induced oral mucositis in mice. Am J Transl Res. 2019;11(8):4789–99.
Xiao XH, Huang QY, Qian XL, et al. Cdc42 promotes ADSC-derived ipc induction, proliferation, and insulin secretion Via Wnt/beta-catenin signaling. Diabetes Metab Syndr Obes. 2019;12:2325–39.
Date S, Sato T. Mini-gut organoids: reconstitution of the stem cell niche. Annu Rev Cell Dev Biol. 2015;31:269–89.
Nam MO, Hahn S, Jee JH, et al. Effects of a small molecule R-spondin-1 substitute RS-246204 on a mouse intestinal organoid culture. Oncotarget. 2018;9(5):6356–68.
Dossena M, Piras R, Cherubini A, et al. Standardized GMP-compliant scalable production of human pancreas organoids. Stem Cell Res Ther. 2020;11(1):94.
Kuijk EW, Rasmussen S, Blokzijl F, et al. Generation and characterization of rat liver stem cell lines and their engraftment in a rat model of liver failure. Sci Rep. 2016;6:22154.
Xie Y, Park ES, Xiang D, et al. Long-term organoid culture reveals enrichment of organoid-forming epithelial cells in the fimbrial portion of mouse fallopian tube. Stem Cell Res. 2018;32:51–60.
Boonekamp KE, Kretzschmar K, Wiener DJ, et al. Long-term expansion and differentiation of adult murine epidermal stem cells in 3D organoid cultures. Proc Natl Acad Sci U S A. 2019;116(29):14630–8.
Lin Y, Fang ZP, Liu HJ, et al. HGF/R-spondin1 rescues liver dysfunction through the induction of Lgr5(+) liver stem cells. Nat Commun. 2017;8(1):1175.
Ishizaki T, Uehata M, Tamechika I, et al. Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol Pharmacol. 2000;57(5):976–83.
Lau H, Kranenburg O, Xiao H, et al. Organoid models of gastrointestinal cancers in basic and translational research. Nat Rev Gastroenterol Hepatol. 2020;17(4):203–22.
Zhou J, Li C, Liu X, et al. Infection of bat and human intestinal organoids by SARS-CoV-2. Nat Med. 2020;26(7):1077–83.
Yip H, Tan CW, Hirokawa Y, et al. Colon organoid formation and cryptogenesis are stimulated by growth factors secreted from myofibroblasts. PLoS One. 2018;13(6):e199412.
Zhang C, Guo H, Yang C, et al. The biological behavior optimization of human periodontal ligament stem cells via preconditioning by the combined application of fibroblast growth factor-2 and A83–01 in in vitro culture expansion. J Transl Med. 2019;17(1):66.
Gargett CE, Gurung S, Darzi S, et al. Tissue engineering approaches for treating pelvic organ prolapse using a novel source of stem/stromal cells and new materials. Curr Opin Urol. 2019;29(4):450–7.
Aoki H, Yamashita M, Hashita T, et al. Efficient differentiation and purification of human induced pluripotent stem cell-derived endothelial progenitor cells and expansion with the use of inhibitors of ROCK, TGF-beta, and GSK3beta. Heliyon. 2020;6(3):e3493.
Hosseini V, Maroufi NF, Saghati S, et al. Current progress in hepatic tissue regeneration by tissue engineering. J Transl Med. 2019;17(1):383.
Orkin RW, Gehron P, McGoodwin EB, et al. A murine tumor producing a matrix of basement membrane. J Exp Med. 1977;145(1):204–20.
Gjorevski N, Sachs N, Manfrin A, et al. Designer matrices for intestinal stem cell and organoid culture. Nature. 2016;539(7630):560–4.
Lindborg BA, Brekke JH, Vegoe AL, et al. Rapid induction of cerebral organoids from human induced pluripotent stem cells using a chemically defined hydrogel and defined cell culture medium. Stem Cells Transl Med. 2016;5(7):970–9.
Verstegen M, Willemse J, van den Hoek S, et al. Decellularization of whole human liver grafts using controlled perfusion for transplantable organ bioscaffolds. Stem Cells Dev. 2017;26(18):1304–15.
Wei G, Wang J, Lv Q, et al. Three-dimensional coculture of primary hepatocytes and stellate cells in silk scaffold improves hepatic morphology and functionality in vitro. J Biomed Mater Res A. 2018;106(8):2171–80.
Eiraku M, Takata N, Ishibashi H, et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472(7341):51–6.
Hu Y, Sui X, Song F, et al. Lung cancer organoids analyzed on microwell arrays predict drug responses of patients within a week. Nat Commun. 2021;12(1):2581.
Muguruma K, Nishiyama A, Kawakami H, et al. Self-organization of polarized cerebellar tissue in 3D culture of human pluripotent stem cells. Cell Rep. 2015;10(4):537–50.
Reilly K, Ellis LA, Davoudi HH, et al. Daphnia as a model organism to probe biological responses to nanomaterials-from individual to population effects via adverse outcome pathways. Front Toxicol. 2023;5:1178482.
Natarajan V, Simoneau CR, Erickson AL, et al. Modelling T-cell immunity against hepatitis C virus with liver organoids in a microfluidic coculture system. Open Biol. 2022;12(3):210320.
Huang P, Zhang L, Gao Y, et al. Direct reprogramming of human fibroblasts to functional and expandable hepatocytes. Cell Stem Cell. 2014;14(3):370–84.
Mun SJ, Lee J, Chung KS, et al. Effect of microbial short-chain fatty acids on CYP3A4-mediated metabolic activation of human pluripotent stem cell-derived liver organoids. Cells. 2021;10(1):126.
Salmon I, Grebenyuk S, Abdel FA, et al. Engineering neurovascular organoids with 3D printed microfluidic chips. Lab Chip. 2022;22(8):1615–29.
Lim J, Kwang LG, Ho N, et al. Hepatocellular carcinoma organoid co-cultures mimic angiocrine crosstalk to generate inflammatory tumor microenvironment. Biomaterials. 2022;284:121527.
Zandi SR, Youhanna S, Keulen J, et al. Bioengineered pancreas-liver crosstalk in a microfluidic coculture chip identifies human metabolic response signatures in prediabetic hyperglycemia. Adv Sci (Weinh). 2022;9(34):e2203368.
Wang Y, Wang H, Deng P, et al. Modeling human Nonalcoholic Fatty Liver Disease (NAFLD) with an organoids-on-a-chip system. ACS Biomater Sci Eng. 2020;6(10):5734–43.
Deng J, Wang ES, Jenkins RW, et al. CDK4/6 inhibition augments antitumor immunity by enhancing t-cell activation. Cancer Discov. 2018;8(2):216–33.
Dijkstra KK, Cattaneo CM, Weeber F, et al. Generation of tumor-reactive t cells by co-culture of peripheral blood lymphocytes and tumor organoids. Cell. 2018;174(6):1586–98.
Neal JT, Li X, Zhu J, et al. Organoid modeling of the tumor immune microenvironment. Cell. 2018;175(7):1972–88.
Sahin U. Studying tumor-reactive t cells: a personalized organoid model. Cell Stem Cell. 2018;23(3):318–9.
Chakrabarti J, Koh V, So J, et al. A preclinical human-derived autologous gastric cancer organoid/immune cell co-culture model to predict the efficacy of targeted therapies. J Vis Exp. 2021;173:e61443.
Zhou G, Lieshout R, van Tienderen GS, et al. Modelling immune cytotoxicity for cholangiocarcinoma with tumour-derived organoids and effector T cells. Br J Cancer. 2022;127(4):649–60.
Kucukkose E, Heesters B A, Villaudy J, et al. Modeling resistance of colorectal peritoneal metastases to immune checkpoint blockade in humanized mice. J Immunother Cancer. 2022;10(12):e005345.
Fiorini E, Veghini L, Corbo V. Modeling cell communication in cancer with organoids: making the complex simple. Front Cell Dev Biol. 2020;8:166.
Ohlund D, Handly-Santana A, Biffi G, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med. 2017;214(3):579–96.
Acknowledgements
None.
Funding
This work was supported by Research Projects from the Science and Technology Commission of Shanghai Municipality (No. 21JC1401200).
Author information
Authors and Affiliations
Contributions
Qiang Gao and Chen Sang are responsible for project selection, data collection, and manuscript drafting. Jian Lin and Shuyi Ji are responsible for revision of the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Gao Qiang is one of the editors of Clinical Cancer Bulletin. Other authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sang, C., Lin, J., Ji, S. et al. Progress, application and challenges of liver organoids. CCB 3, 7 (2024). https://doi.org/10.1007/s44272-024-00012-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44272-024-00012-0