Abstract
Surface Enhanced Raman Scattering (SERS) is a pivotal detection technology that identifies molecular spectral signals at the single-molecule level. Owing to its sensitivity and non-destructive nature, SERS has found extensive applications in diverse fields such as environmental science, medical diagnosis, food safety, and bioanalysis. In recent years, two-dimensional materials have emerged as one prominent novel SERS-active nanomaterials due to their unique physical and chemical properties. This paper provides a comprehensive review of the enhancement mechanism of SERS, substrate preparation, and its applications in biosensing, environmental detection, and food safety. It delves into the widely accepted basic theory and enhancement mechanism of SERS, along with common methods to boost SERS performance, including doping with other elements, applying stress and strain, forming defects, and amorphous processing. This paper examines the current state of research on two-dimensional(2D) materials and their application in the field of SERS. Additionally, it provides an viewpoints on recent advancements in the use of this technology for virus and cancer detection. Lastly, it discusses the future prospects for the development of SERS technology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
SERS is a phenomenon where the spectral intensity of molecular Raman scattering is significantly amplified on certain specialized material surfaces. First discovered by Fleischmann and colleagues Hendra and McQuillan on a rough Ag electrode in 1974 [1], this effect was later proposed by Van Duyne and Creighton in 1977 [2]. SERS, as a single molecule spectroscopy detection technology, can provide insights into material structure and chemical composition at the single molecule level, enabling trace detection of analytes. Currently, due to its sensitivity, speed, and non-destructive nature, SERS technology is garnering attention in numerous fields such as biomedicine, environmental science, chemistry, and food safety. In particular, within the realm of biosensing, SERS presents a promising alternative to traditional detection technologies like colorimetry and immunochromatography because of its specificity, non-destructiveness, and universality (applicable to solid, liquid, and gaseous states). This makes it particularly well-suited for applications in virus detection and tumor identification [3].
Since the inception of SERS technology, scholars across diverse disciplines such as physics, chemistry, and materials science have engaged in the exploration of this highly sensitive detection methods. The predominant focus within SERS research has centered on the advancement of SERS substrates. Initial emphasis was directed towards the utilization of rough precious metal substrates [4]. Numerous investigations on metal substrates have underscored the correlation between SERS properties and factors such as material type, as well as the geometry and size of metal nanoparticles. Given that silver (Ag) exhibits the lowest optical loss in the visible and near-infrared regions, it is regarded as the optimal plasmonic material. However, the roughening of the Ag surface leads to rapid oxidation, diminishing the plasma enhancement effect and subsequently reducing its SERS activity. Gold (Au), another common precious metal, has garnered attention due to its exceptional chemical stability in natural environments. Despite the commendable SERS activity exhibited by both Au and Ag, their high costs impose significant constraints on their practical application [5]. Consequently, researchers have redirected their attention towards copper (Cu) and aluminum (Al) as potential substitutes for Au and Ag. Pure Cu and Al metals are inherently unstable under natural conditions, prone to rapid oxidation. Hence, alloys have emerged as promising candidates for SERS substrates. The Surface Plasmon Resonance (SPR) frequency varies with the degree of alloying. AuxAg(1-x) alloy nanoparticles, for instance, exhibit enhancement in the UV–VIS spectrum. As the particle size increases, the plasmon resonance wavelength experiences a redshift [6]. Comparative studies have revealed that the Raman enhancement factor of Au nanoparticles is only 106, significantly lower than the Raman signal at the single-molecule level [7]. Consequently, the optimization of the plasma electric field is typically accomplished through modifications to the geometry, size, and structure of the plasma nanostructure [8]. This is illustrated by Wiley et al., who demonstrate the impact of altering the binding shape of Ag nanostructures, conclusively establishing the dependence of plasma properties on the geometric shape of metal materials [9].
The exorbitant cost of precious metals, along with the uneven distribution of hot spots and challenges associated with storage, has significantly constrained the practical application of SERS technology. In a seminal development in 1982, Loo and colleagues employed the semiconductor material TiO2 as a working electrode [10], marking the inaugural observation of SERS on a semiconductor substrate. This groundbreaking discovery sparked heightened scholarly interest, leading to extensive research on semiconductor substrates exhibiting SERS activity. A plethora of new semiconductor materials with substantial SERS efficacy have since emerged. In 1983 [11], Yamada et al. achieved SERS spectra of pyridine adsorbed on semiconductor single crystals NiO and TiO2 at room temperature. The characteristic Raman peaks of pyridine, obtained through adsorption on the surfaces of NiO and TiO2, provided evidence of an interaction between the crystal face and the semiconductor single crystal atom. Concurrently, Potts et al. manipulated the surface roughness of PbTe to 0.1–0.3 µm, resulting in the observation of SERS enhancement phenomena for amorphous carbon adsorbed on its surface [12]. This underscored the existence of SERS phenomena on semiconductor surfaces with varying roughness. Nevertheless, semiconductor substrates suffer from lower sensitivity compared to precious metal substrates. Consequently, current research endeavors focus on the development of materials with heightened SERS activity and the refinement of strategies for optimizing semiconductor SERS activity.
In recent years, two-dimensional materials, spearheaded by graphene, have emerged as promising SERS substrates. Graphene, characterized by a zero-band gap nonpolar two-dimensional structure with abundant excited electrons near the Highest Occupied Molecular Orbital (HOMO) and Lowest Unoccupied Molecular Orbital (LUMO) levels, exhibits a robust charge transfer effect. Hexagonal boron nitride (h-BN), a graphene-like wide-band gap 2D material, enhances SERS performance through dipole–dipole interactions with substance molecules. Transition metal disulfides (TMDS) [13], another class of two-dimensional materials, exemplified by hexagonal MoS2 with a honeycomb structure resembling graphite [14], possess a band gap between graphene and h-BN. Most TMDS enhance SERS activity through charge transfer resonance and coordination via dipole–dipole interactions [15]. Furthermore, MXene has gained popularity as a contemporary two-dimensional material [16]. Its unique metallicity contributes to SERS enhancement through a combination of electromagnetic and chemical enhancements. Presently employed in the SERS field, MXene materials selectively enhance positively charged molecules such as methylene blue (MeB) and crystal violet (CV) due to their negatively charged surface [17].
Compared to zero-dimensional, one-dimensional, and three-dimensional bulk materials, 2D materials possess a significant advantage in forming complexes with probe molecules owing to their constrained atomic coordination number on the surface. The presence of abundant defect states on the surface further facilitates the facile regulation of the energy level structure of these materials. This heightened regulation enhances the tendency for charge transfer between two-dimensional materials and substance molecules, consequently leading to an amplified charge transfer resonance. Additionally, the surface of 2D materials is inherently modifiable and conducive to assembly, thus facilitating the integration of various functional groups or the construction of P-N junctions to further optimize SERS properties. As the repertoire of two-dimensional materials continues to expand, their physical and chemical properties undergo systematic optimization, resulting in a continuous enhancement of SERS activity. Consequently, two-dimensional materials have emerged as a focal point in SERS substrate research.
Hence, this paper comprehensively reviews and discusses the advancements in research and applications of two-dimensional (2D) materials in the field of SERS. The schematic representation of the article structure is delineated in Fig. 1. The initial sections provide an overview of the evolution of SERS technology alongside the development of 2D materials. Two widely acknowledged enhancement mechanisms, namely electromagnetic enhancement and chemical enhancement, are thoroughly examined. The discussion extends to encompass the preparation methodologies of 2D materials, an evaluation of SERS sensitivity pertaining to these materials, and an exploration of common approaches to enhance the SERS activity of these materials. Subsequently, the paper reviews the diverse SERS applications of select 2D materials in areas such as biomolecular capture, virus detection, and tumor diagnosis. Finally, the concluding sections offer insights into the anticipated future trends and prospects in this rapidly evolving field.
2 Basic theory of SERS technology
Li analyzed SERS enhancement from mesoscopic and macroscopic strategies [18], and expressed the total Raman scattering intensity of material molecules on SERS substrate as follows [19]:
Here, the symbol A denotes the Raman signal acquisition efficiency of the optical detection system, while \({I}_{0}({r}_{0},\omega )\) represents the incident light intensity, both of which are contingent upon external detection conditions. The term \(\alpha ({\omega }_{R},\omega )\) denotes the polarizability tensor associated with the vibration of the material molecule, and \(G({r}_{0})\) signifies the enhancement factor of the local field, proportionate to the fourth power of the electromagnetic field strength surrounding the material molecule. Consequently, augmenting the Raman scattering intensity of material molecules on the SERS substrate involves elevating the polarization tensor of material molecules and intensifying the electromagnetic field strength. As a result, two universally recognized SERS enhancement mechanisms have been formulated to elucidate this nonlinear optical enhancement phenomenon, namely the Electromagnetic Enhancement Mechanism (EM) and the Chemical Enhancement Mechanism (CM).
Three contributions that play a major role on the SERS enhancement factor have been identified so far: (1) the plasmon resonance of the noble metal nanoparticle surface; (2) the charge transfer resonance between the molecule of matter and the metallic conduction band near the Fermi level; (3) the resonance of the adsorbed molecules themselves, which is not only essential to enhance the Raman signal intensity of the molecules but also makes single molecule effect easier to be observed. These three components are often treated as independent contributions to the overall effect, which means that one can choose which resonance participates in the SERS process by adjusting experimental parameters. But these three resonance effects seem to be completely different and their contribution to the overall enhancement varies greatly. Their SERS enhancement effect often depends on the properties of nanoparticles, the properties of molecules themselves and the excitation wavelength of lasers. Lombardi et al. [20]. integrated surface plasmon resonance, charge transfer resonance between substrate and molecules at Fermi level and molecular resonance occurring at excitation wavelength together to propose an expression for SERS in 2008. It was an expression for evaluating the intensity of surface enhanced Raman scattering by summing up the polarizability \({R}_{mol-CT}(\omega )\) of all molecules in a system of substrate-matter molecules, where the intensity of Raman scattering is proportional to square of polarizability \({\left|{R}_{mol-CT}(\omega )\right|}^{2}\) of all molecules. To obtain an expression for Raman intensity around multiple nanoparticles, we follow Albrecht’s procedure to introduce Herzberg-Teller vibration coupling into expression of polarizability, thus coupling three resonances together [21]. So the polarizability of molecular-semiconductor complex system with Herzberg-Teller vibration coupling introduced can be described as [22]:
In this, \({E}_{sp,\beta }\) represents the magnitude of the electric field generated on the surface of the SERS substrate, and \({Q}_{K}\) is the normal vibration mode of the molecule. Molecular resonance (\({\mu }_{mol}\)) and charge transfer resonance (\({\mu }_{CT}\)) are achieved by mutual coupling through Herzberg-Teller coupling constants (\({h}_{mol-CT}\)). \({\upvarepsilon }_{0}\) refers to the real part of the dielectric constant of the surrounding environmental medium, while \({\varepsilon }_{1}\) and \({\varepsilon }_{2}\) refer to the real and imaginary parts of the dielectric constant of the SERS substrate, respectively. \(\chi\) is a constant factor for the deviation of the substrate from spherical geometry. The denominator of the above formula contains three parts to jointly contribute to SERS intensity. When the frequency of the incident light is close to a resonance frequency of the composite system, SERS enhancement must involve the corresponding part. When \({\upvarepsilon }_{1}\left(\upomega \right)=-2{\varepsilon }_{0}\) surface plasmon resonance plays a role in SERS enhancement for semiconductor substrates; when \(\omega ={\omega }_{CT}\), charge transfer resonance between probe molecules and substrates plays a role in SERS enhancement for semiconductor substrates; \(\omega ={\omega }_{mol}\) represents molecular resonance. Therefore, when a determined incident laser frequency is provided, one or more resonances in the molecule-semiconductor substrate system will be involved. Theoretically, to obtain a strong SERS signal, one needs to choose the optimal material molecule-semiconductor substrate system and experimental parameters to satisfy the SERS selection rule. This allows the system to generate molecular resonance, charge transfer resonance, and plasmon resonance at a certain excitation wavelength, thereby realizing three quasi-resonance Raman effects, namely, \({\lambda }_{mol}\approx {\lambda }_{laser;}{\lambda }_{CT}\approx {\lambda }_{laser;}{\lambda }_{plasmon}\approx {\lambda }_{laser}\). Therefore, this expression well unifies the above three resonance effects, providing a theoretical basis for coupled resonance strategies. It not only determines the source of SERS enhancement and the parameters controlling enhancement but also provides a systematic method and measure for how to synergistically act on the three resonances [23].
2.1 Electromagnetic enhancement mechanism
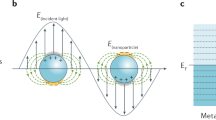
Electromagnetic enhancement refers to the phenomenon where, when the frequency of the incident laser closely aligns with the frequency of the surface plasma vibration of the substrate material, a highly localized electric field forms on the metal particles or rough surface. This localized electric field significantly amplifies the Raman signal of the analyzed material in the region [24]. The essence of this enhancement lies in SPR occurring on the substrate surface. SPR predominantly manifests in two types of metal interfaces: surface plasmons (SPs) that propagate along the metal–dielectric interface, known as propagating plasmons, and local SPs that are confined around metal nanoparticles. As depicted in Fig. 2a, the free electrons on the surface of metal nanoparticles undergo vibration, inducing localized plasma and charge polarization. Studies demonstrate that molecules within the range of electric fields, approximately 10–100 Å on the substrate surface, can be excited by these electric fields [24]. The electromagnetic enhancement factor can exceed 1011, establishing the electromagnetic enhancement mechanism as a potent and enduring enhancement effect mechanism. The plasma frequency, expressed as \({\omega }_{R}={\left(\frac{4\pi N{e}^{2}}{{\varepsilon }_{\alpha }{m}^{*}}\right)}^{1/2}\), is central to this mechanism. Here, N represents the carrier concentration represented by electrons or holes, and \({m}^{*}\) denotes the effective mass of the carrier. \({\omega }_{R}\) is proportionate to the carrier concentration N [25]. Given the inherently high carrier concentration in metals, early studies on electromagnetic enhancement primarily focused on precious metals. Recent advancements reveal that the carrier concentration of semiconductors can be widely controlled by modifying the material characteristics through doping, constructing heterogeneous structures, and employing other methods [26]. Apart from the material's intrinsic characteristics, the frequency of plasma vibration and the resulting electric field intensity are also influenced by the size and morphology of the material. Both metal and semiconductor materials can modulate the magnitude of their electromagnetic enhancement by effectively adjusting their carrier concentration [27]. To comprehensively understand the electromagnetic enhancement effect of surface plasmas in various nanostructures, commonly utilized methods include the discrete dipole method (DDA), finite difference time domain method (FDTD), and finite element method (FEM), which accurately simulate the electric field distribution of material nanostructures.
When nanoparticles of different or the same geometry are gathered together, a combination will be formed. When these nanoparticles form dimers, trimers or interstitial regions of dimers symmetrically placed by metal nano-triangles, there is a significantly enhanced LSPR electromagnetic field. Considering that the electromagnetic field has strong spatial localization and strong enhancement phenomenon on signals of matter molecules, this region is called "hot spot". The working principle of hot spot area is shown in Fig. 2b [28]. Hot spots usually appear in areas/regions where the connection of metal nanoparticles, gap, small radius of curvature or highly curved, such as: the tip of nanostructures, inflection points (lightning rod effect) The speciality of the emergence area determines the non-uniformity of hot spot distribution. Therefore, in the detection of noble metal SERS substrates, it is often that hot spots can greatly enhance molecular in hot spot area without falling into hot spot area, the Raman signal of substance molecules that do not fall into hot spot area will not be greatly enhanced. Although noble metal SERS substrates with hot spot effect can realize high sensitivity detection of substance molecules, some even at the single molecule level, noble metal substrates face problems such as poor stability, poor repeatability and poor biocompatibility which are difficult to solve [7]. And just the opposite of noble metal substrates, semiconductor SERS substrates just can overcome the defects of noble metal substrates in practical applications, thereby further expanding the application field of SERS technology [29].
2.2 Chemical enhancement mechanism
It is well established that the Electromagnetic Enhancement Mechanism (EM) primarily elucidates the high SERS activity observed in precious metals. However, semiconductor materials inherently possess a low carrier concentration [30]. For instance, the intrinsic carrier concentration of silicon (Si) at 300K is merely 1.5 × 1010 cm−3, whereas the carrier concentration for gold (Au) is detected at 1022 cm−3 [31]. Figure 3a illustrates the corresponding surface plasmon frequencies (\({\omega }_{R}\)) and carrier densities for nanoparticles of various materials and sizes [32]. For noble metal nanoparticles, the carrier density approximates to a range of 1022 to 1023 cm−3, with corresponding plasmon resonance frequencies situated in the near-infrared and visible light regions. In contrast, for doped semiconductor nanoparticles, the carrier density approximates to a range of 1016 to 1021 cm−3, with corresponding plasmon resonance frequencies located in the terahertz and near-infrared regions. This is attributed to the fact that heavily doped semiconductors possess excited electronic states near the Fermi level [32].
a The relationship between carrier density and plasma frequency of metal, doped semiconductor and degenerate doped semiconductor [32]; Energy level transition diagram of b Metal-molecular system and c Semiconductor molecular system
Consequently, most semiconductor materials lack free electrons on the surface necessary for the formation of a robust plasmon resonance. Simultaneously, semiconductor substrates exhibit selectivity, selectively enhancing the Raman peak of specific molecules. For example, under identical experimental conditions, the Raman cross sections of nitrogen (N2) and carbon monoxide (CO) molecules are equivalent, yet their enhancement factors differ by 200 times [33]. This inconsistency appears to be correlated with the occurrence of chemisorption and its location on the active adsorption site [34], phenomena that cannot be fully elucidated solely by electromagnetic enhancement. In consideration of these findings, we propose a charge transfer-driven Chemical Enhancement Mechanism (CM) to explain the SERS activity of semiconductor substrates. When a material molecule adsorbs onto the surface of a semiconductor substrate, the material surface or other atoms adsorbed on the surface undergo charge transfer with the probe molecule. This exchange alters the charge density of the material, leading to an increase in molecular polarizability and the Raman scattering interface, thereby amplifying the Raman signal of the molecule.
Up to now, common chemical enhancement mechanisms can be broadly categorized into three main groups: (1) The formation of chemical bonds between semiconductor SERS substrate materials and substance molecules; (2) When the material molecules were adsorbed on the semiconductor SERS material, the surface complex was formed between them, which resulted in the surface complex enhancement; (3) Photoinduced charge transfer occurs when a specific frequency of excitation light irradiates a semiconductor substrate containing molecules [35].
These enhancement mechanisms can be further classified into static chemical enhancement and dynamic chemical enhancement. Chemical bond enhancement and surface complex enhancement mechanisms fall under static chemical enhancement. Chemical bond enhancement aims to increase the effective volume and ground state polarizability of molecules through bonding, consequently augmenting the Raman signal of molecules [36]. Surface complex enhancement involves the formation of complex resonances under the irradiation of incident light, thereby increasing the Raman signal [37]. Both of these mechanisms are categorized as static chemical enhancement. On the other hand, PICT involves a strong coupling between excitation light photons and electrons within the SERS substrate-molecular complex system, leading to enhanced PICT resonance. The prerequisite for this coupling is that the photon energy should be similar to the energy required for the electron transition of the SERS substrate-molecular complex. PICT belongs to dynamic chemical enhancement, which is considered the most crucial enhancement mechanism and is widely employed in interpreting the SERS phenomenon on semiconductor surfaces [38].
In the enhanced charge transfer mechanism, effective charge transfer occurs exclusively when the HOMO and LUMO of the molecule precisely align with the valence band (VB) and conduction band (CB) of the substrate. This alignment enables charge transfer to take place under the excitation light of the corresponding wavelength. There are two directions of charge transfer: charge from substrate to molecule and molecule to substrate.
The direction of charge transfer depends on the properties of the substance molecule. When the substance molecule has a π-space orbital, the charge is likely to transfer from the substrate material to the substance molecule. When the substance molecule does not have a π-space orbital, the charge tends to transfer from the substance molecule to the substrate material. The charge transfer diagram of metal-molecular system and semiconductor-molecular system are shown in Fig. 3b and c. In metal-molecular system, under the excitation of incident light, electrons near Fermi level (EF) absorb energy to become excited state, resulting in electron–hole pair at Fermi level. Due to interaction between molecule and metal substrate, excited electron transfers to LUMO energy level of molecule. Transferred electron exchange energy in LUMO energy level of molecule, which change some vibrational energy levels of substance molecule. At this time, material molecule is in excited state. In addition, Raman scattered photons specific to molecule are released and jump back to metal EF to recombine with hole. For semiconductor-molecular system, taking LUMO from CB of semiconductor to probe molecule as example, main process is: under excitation of incident light, semiconductor electron transits from VB to CB, leaving hole at VB to form electron–hole pair. Due to interaction between semiconductor material and molecule, electrons at CB are transferred to LUMO energy level of matching molecule. Finally, scattered photons dedicated to molecular energy are released and electrons return to semiconductor valence band and hole recombination [13].
The aforementioned charge transfer necessitates certain conditions for successful execution [39]: (1) An interaction between the substrate material and the substance molecules must exist, which determines both the probability of charge transfer and the relaxation time length in the molecular orbital; (2) T The energy level of the substrate material and the molecule must be compatible; (3) The energy of the excitation light must reach the minimum required for the charge transfer process. Conversely, an energy level structure that is too distant makes the charge transfer process challenging to achieve. Generally, it is difficult to completely distinguish between electromagnetic enhancement mechanisms and chemical enhancement mechanisms, as they often operate together on the same SERS substrate [40].
3 2D SERS-active materials and their preparation methods
Two-dimensional materials, characterized by their planar nature and single or multiple atomic layers in thickness, are emerging as promising candidates for SERS substrate research. This is primarily due to their rich electronic states and intricate band structures. Presently, there is a growing interest in two-dimensional materials such as graphene, graphene-like structures, transition metal sulfides, MXene materials, and black phosphorus(BP) [41]. Notably, the SERS sensitivity of certain of these materials has reached levels comparable to, if not exceeding, that of noble metals. Consequently, they hold significant potential for applications in areas including food safety, environmental monitoring, and biosensing [42].
3.1 Chemical vapor deposition
Chemical vapor deposition (CVD) is a process in which gaseous substances are chemically reacted on a solid surface at a certain temperature and a solid deposition film is formed on the surface [43]. It is a simple method to synthesize one-dimensional nanomaterials with high crystallinity and high purity.
Due to the continuous advancements in CVD technology, the field of materials preparation has undergone significant maturation. Graphene, an exceptional 2D material, exhibits remarkable chemical stability when prepared as a single layer through the conventional CVD method, as shown in Fig. 4a [44]. This material retains its superior SERS activity even under conditions of high concentration hydrochloric acid (35% ~ 37%) and temperatures up to 400 °C. In addition, researchers have enhanced the SERS platform by depositing thin films on substrate surfaces. For instance, Savtha et al. developed the Graphene-based Surface Enhanced Raman Scattering (G-SERS) platform by depositing silver onto a SiO2 substrate [45]. The detection limit for the R6G probe molecule synthesized on this platform using monolayer graphene was found to be 10–14 M, with an SERS enhancement factor of 9.48 × 108. However, Ayhan et al. used a similar approach to prepare modified graphene oxide [46], but their findings indicated that the detection limit for the R6G probe molecule was slightly higher at 10–13 M. This discrepancy is attributed to the incorporation of the noble metal Ag, which enhances both the electric field and charge transfer efficiency near graphene. Additionally, Zhao et al. achieved the successful synthesis of two-dimensional GaN crystals under the assistance of a mixed Ar and H2 gas environment, operating at 1080 °C for 5–10 min [47]. In comparison to their three-dimensional bulk GaN counterparts, chemically vapor-deposited two-dimensional GaN crystals exhibit enhanced SERS activity, attributed to the increased electron state density and amplified charge transfer resonance. As illustrated in Fig. 4b [47], the detection limit of the 2D-GaN crystal can reach 10–7 M, with an EF of 105. Additionally, Sodium Chloride (NaCl) stands out as a widely adopted additive, besides serving as protective atmospheres. Song et al. ingeniously employed NaCl as a catalyst to lower the melting point of niobium oxide, yielding sizable ultrathin NbS2 flakes (> 160µm). As depicted in Fig. 4c [48], these meticulously prepared NbS2 flakes have a SERS detection limit for MeB as low as 10–14 M, enabling them to proficiently differentiate between different types of red wines. Moreover, NaCl also proves invaluable as an auxiliary agent, facilitating the controlled synthesis of large-area NbSe2 flakes with varying layer numbers, as illustrated in Fig. 4d [49]. Throughout the CVD process, the incorporation of higher selenium and hydrogen content ensures the growth of few-layer NbSe2, while an increased supply of selenium effectively reduces the presence of selenium vacancies, thereby enhancing the stability of the grown NbSe2 in ambient air. Both experimental and theoretical analyses have unequivocally demonstrated that R6G's detection limit on 6L-NbSe2 SERS substrates can astonishingly reach an unprecedented 5 × 10–16 M.
a Sulfides are grown by atmospheric chemical vapor deposition (AP-CVD) [44]; b Raman spectra of CV solution on 2D GaN with various concentrations [47]; C The Raman spectra of MeB were obtained on 2 nm thick NbS2 by immersion in 1 × 10−6 ~ 1 × 10−16 M solution with different concentrations [48]. Inset: Raman intensity diagram of MeB (1 × 10−6 M) 1621 cm−1 peak on a large NbS2 sheet; d Raman intensity of R6G molecular characteristic peaks (1361 and 1646 cm−1) on NbSe2 with different layers [49]
Furthermore, CVD technology has opened up a new avenue for synthesizing the increasingly popular MXene materials, resulting in the creation of novel MXenes like Zr2CCl2, Zr2CBr2, and pure phase Ti2NCl2. It has also introduced a fresh pathway for nitrogen incorporation within MXene. Iravani et al. harnessed CVD-synthesized MXene for the detection and monitoring of ultraviolet light [50], underscoring its immense potential in skincare and cancer prevention devices. Presently, as the MXene portfolio continues to expand, it is gradually transitioning from a planar structure to spherical MXene vesicles. This top-down or bottom-up synthesis route provides precise control over the MXene synthesis process [51]. This precision not only governs the phase and crystal structure of MXene, but also generates a diverse range of highly crystalline MXene members, thereby significantly broadening the MXene family's scope.
3.2 Hydrothermal method
The hydrothermal reaction is mainly controlled by medium and low temperature liquid phase, and the reaction and crystal growth are controlled by changing the temperature and time of hydrothermal reaction.
Utilizing hydrothermal reactions as a foundation, a range of strategies, including the addition of surfactants, introduction of dopant elements, hydrogenation, and adjustments in reaction parameters, can be employed to exert control over the products of hydrothermal reactions, ultimately aiming to explore their SERS activity. Hydrogenation emerges as a viable means to enhance SERS performance. Yang et al. integrated hydrothermal synthesis with hydrogenation processes, resulting in a remarkable three-order-of-magnitude improvement in the SERS performance of TiO2 products. Hydrogenated TiO2 displayed a detection limit of 10–7 M for R6G and an EF of 1.20 × 106 [52], comparable to noble metal Ag substrates. Polyethylene glycol (PEG), a commonly employed surfactant, can effectively modulate grain growth activity during the reaction process. Liang et al. successfully synthesized samples with distinct growth orientations [53], designated as BCN-30, BCN-60, and BCN-90, by manipulating the percentage of PEG (30%, 60%, 90%) in the synthesis. The morphology of the generated BCN nanosheets is shown in Fig. 5c [54]. When subjected to SERS testing using the R6G probe molecule(Fig. 5d), BCN-60 achieved an impressive detection limit of 10–8 M and an EF of 5.34 × 105. Meanwhile, BCN-60 achieves a SERS detection limit for malachite green as low as 10–8 M (Fig. 5f). Additionally, the introduction of dopant elements during hydrothermal reactions to modify the energy level structure of materials represents another potent strategy to enhance SERS activity. Zhang et al. introduced Ru and O dopants into MoS2 (MSORx) during the hydrothermal process, as shown in Fig. 5a [55]. This doping of Ru and O elements generated additional active sites, facilitating accelerated charge transfer between molecules and the substrate system. Consequently, this enabled highly sensitive detection of bilirubin with a LOD of 10–10 M and an EF of 1.7 × 106 (Fig. 5e) [55]. Parameters such as temperature and reaction duration during hydrothermal reactions also exert a notable influence on the reaction kinetics governing crystal growth. Li et al. successfully synthesized MoS2 with varying interlayer distances by adjusting the temperature and duration of hydrothermal reactions (Fig. 5b) [54], thereby elucidating the interlayer distance's impact on SERS performance. The corresponding enhancement factor statistics of different interlayer distances are shown in Fig. 5g, and its optimal EF is 5.31 × 105. In conclusion, hydrothermal reactions prove to be an effective method for material synthesis, and their SERS activity can be further enhanced through the combined application of various complementary techniques [53].
a Schematic illustration of the synthetic pathways for MoS2 and MSOR1 NFs with different structures [55]; b SEM image of MoS2 NFs; c TEM image of BCN-60 showing its sheeted nature [54]; d Schematic illustration for the synthesis of h-BN and BCN nanosheets [54]; e Raman spectra of MB at concentrations ranging from 10−3 to 10−9 M collected on MSOR1 NFs [55]; f SERS spectra of the detected malachite green of different concentrations by employing BCN-60 as a SERS substrate [53]; g EFs of the MoS2-0.62, MoS2-0.87, and MoS2-1.12, probed with 4-MBA, 4-MPy, and 4-ATP [53]
3.3 Sintering and other methods
In addition to CVD and hydrothermal methods, other prevalent techniques for the preparation of two-dimensional materials encompass sintering and etching processes. Sintering is a process of converting a powder into a dense body at high temperatures (below the melting point) [56]. The etching method involves the utilization of potent acids and alkalis, such as HF and hydrochloric acid, as etching agents to remove carbon from the MAX phase, subsequently forming the MXene phase.
Calcination have predominantly been employed in the preparation of amorphous materials. Guo et al. successfully synthesized amorphous ZnO NCs by calcination as shown in the Fig. 6 (b) [57], confirming that the introduction of amorphous characteristics results in an exceptionally high SERS enhancement factor. This marked the first instance of such pronounced SERS activity observed in amorphous two-dimensional semiconductor nanomaterials. Consequently, amorphization has become one of the means to enhance SERS activity in two-dimensional materials. Subsequently, Wang et al. conducted a comparative study between crystalline and amorphous 2D-TiO2 prepared through annealing processes at different temperatures [58]. By comparing their SERS performance, it was revealed that the high surface potential induced by the amorphous treatment strongly attracts electron clouds, leading to a significant interface charge transfer. The amorphous 2D-TiO2 exhibited high SERS activity with EF of up to 1.86 × 106. The synergistic process of calcination and exfoliation has the potential to disrupt the inherent flat surface of a material, thereby facilitating the grafting of additional functional groups and the creation of defect sites. Therefore, Song et al. employed an innovative strategy that combined exfoliation and calcination to successfully fabricate ultrathin WO3 nanosheets, which exhibited abundant defect sites on their surfaces [48]. These defect sites have the capacity to augment the charge carrier density while simultaneously inducing substrate-molecule charge transfer interactions. This dual effect not only ensures reproducibility but also enhances the sensitivity of SERS by over 100 times.
a Schematic illustration of the preparation of MXene materials [59]; b A schematic of the fabrication of a- and c-ZnO NCs [57]; c Raman spectra of MeB with three different concentrations, 10−6 M, 10−7 M and 10−8 M on Nb2C NSs substrates [60]; d Raman spectra of MV with three different concentrations of 10−5 M, 10−6 M and 10−7 M on Ta2C NSs substrates [60]
Since the emergence of Ti3C2Tx MXene materials in 2011 [61], the unique electronic structure, tunable work functions, and high carrier concentration of MXene have rapidly garnered significant research interest. Moreover, the majority of MXene are currently synthesized by etching MAX phases to obtain the desired products. The formula for MAX phases is Mn+1AXn, where M and X represent elements similar to those in MXene, while A primarily denotes a group of IIA or IVA elements. Given that most of the M-A bonds in MAX phases can be quickly etched away, and new M-T bonds can be established, the most commonly used etchant is a fluorine- or chlorine-containing acidic solution. However, MXene sheets obtained through one-step etching remain tightly bound together through interlayer van der Waals forces, necessitating a secondary chemical exfoliation. To date, molecular intercalation using intercalants like Li+, tetrabutylammonium hydroxide, tetramethylammonium hydroxide (TMAOH), and dimethyl sulfoxide (DMSO), in conjunction with multiple washing steps, is commonly employed to create stable MXene suspensions for material preparation [62]. Considering the substantial surface area and atomic coordination number deficiencies of MXene materials, they readily adsorb probe molecules, while their high electronic state density at the Fermi level makes them highly promising for SERS applications. In addition to these common two-dimensional materials, MXene (transition metal carbides, nitrides, and carbonitrides) has emerged as a novel two-dimensional material with atomic thickness and metallic properties. Its outstanding characteristics, including high conductivity, hydrophilicity, tunable electronic structure, and high carrier concentration, have piqued the interest of researchers across various fields. Lan et al. by controlling the pH during the etching process and selecting tetrabutylammonium hydroxide (TPAOH) as an intercalant, successfully obtained different-layered V2C MXene [63], which exhibited a SERS sensitivity of 10–7 M. Similarly, to investigate the influence of different layer numbers and interlayer distances on SERS performance, Peng et al. synthesized Nb2C and Ta2C MXene with varying interlayer distances through a two-step etching process by adjusting etching time and concentration, as shown in Fig. 6a [59]. SERS tests were performed at the optimal resonant excitation wavelength of 532 nm (Fig. 6c and d) [60], and the SERS sensitivities of Nb2C and Ta2C were 3.0 × 106 and 1.4 × 106, respectively, with detection limits of MeB of 10–8 M, 10–7 M, respectively. Due to the complexity of the two-step etching process, which requires multiple deionized water and anhydrous ethanol washes, He et al. proposed a one-step etching method to prepare highly crystalline multi-layered MXene nanosheets without the need for ultrasonication or organic solvent delamination [60]. This one-step etching method successfully prepared TiVC MXene films as SERS substrates, with a detection limit of 10–12 M and an EF of 1012 for R6G.
In recent years, there has been rapid progress in the utilization of 2D materials within the realm of SERS, with the emergence of numerous highly SERS-active 2D materials. As fabrication techniques continue to advance, traditional methods such as CVD, hydrothermal synthesis, annealing, and etching have undergone further refinement. This has led to the production of materials with diverse morphologies and crystalline structures, thereby expanding the repertoire of materials applicable for SERS technology.
4 Application of 2D materials in SERS field
Compared to other materials, 2D materials offer superior uniformity, stability, and biocompatibility. These attributes have been instrumental in advancing SERS technology. Notably, 2D materials can be chemically modified to fine-tune their biocompatibility and electrical properties, thereby enhancing charge transfer efficiency between substrates and molecules. This has significantly facilitated the use of SERS technology in areas such as biosensing, food safety, and environmental monitoring [64].
4.1 The application of SERS technology in biosensing
The application of SERS technology in biosensing mainly focuses on the detection and bioimaging of biomolecules, especially in the detection of biomolecules such as peptides, proteins, tumor markers and some common antibiotics [65]. Common SERS detection methods mainly include direct detection and indirect detection. Direct detection method, namely SERS labeling free detection method, is to let the substance molecule itself directly interact with the SERS substrate to obtain the spectral information of the molecule. This method has the advantage of simple and efficient direct analysis of biomolecular spectra. But the actual biological environment is very complex, there is a certain interaction between various biomolecules, and there is a superposition of Raman summit of different biomolecules. Therefore, SERS marker-free detection method can hardly meet the sensitivity requirement of actual clinical detection. The indirect detection method used probe molecules with strong signal and unique characteristic peak as SERS markers, so as to achieve the detection of biomolecules under biochemical environment [66].
4.1.1 Application of SERS in virus detection
Virus can rapidly and even explosively increase in human body, which poses a great threat to human life and health [67]. At the same time, the virus itself has the characteristics of easy mutation, rapid transmission, immune escape, hidden transmission and so on. Once the virus spreads on a large scale, it will certainly bring bad effects on human society. Therefore, early detection, early isolation and early treatment can fundamentally solve this problem. The good biocompatibility of 2D materials combined with the sensitive characteristics of SERS technology has great application potential in the field of virus detection [68].
The large-scale outbreak of the novel coronavirus in 2020 has attracted wide attention to the methods and technologies for the efficient identification and detection of virus particles [69]. As shown in Fig. 7a [70], Peng et al. realized a two-step diagnosis of SARS-CoV-2 virus particles by SERS technology. The SERS signal recognition criteria of SARS-CoV-2 S protein and RNA were used to diagnose the infectivity of SARS-CoV-2. The detection limit of SARS-CoV-2 S protein using the prepared micron-sized SnS2 can reach 10–14 M (Fig. 7b) [70]. This method can accurately identify noninfectious lytic SARS-CoV-2 virus bodies in real environments, which cannot be done by current PCR methods. Building on these findings, Li et al. developed a novel semiconductor SERS substrate, which could be significantly manipulated by light through the integration of SiO2/Si into the SnS2 substrate [71]. The prepared fabry-p type substrate significantly improved the SERS activity of the SnS2 substrate. At the same time, this substrate helped to reduce the detection limit of SARS-CoV-2S protein, so that its SERS LOD can reach an astonishing 10–16 M (Fig. 7c) [71]. This study provides practical strategies for biosensing applications in the field of semiconductor substrate SERS. The characteristics of two-dimensional materials combined with noble metals also show fascinating advantages in virus detection. Lin et al. The synthesized Au NSs were designed as SERS sensing chips that could distinguish SARS-CoV-2 RNA from other RNA molecules [72]. The integration of internal standard molecules, 4MBA, was able to quantitatively evaluate the real-time Raman signal fluctuations in probe molecules. As shown in Fig. 7e [73], the SERS sensing chip achieved a dynamic detection range of 10–6 M ~ 10–12 M for SARS-CoV-2 S protein in human saliva, with a minimum detection threshold of 7.61 × 10–14 M. Kaladharan achieved rapid, sensitive detection of SARS-CoV-2 by coating Ag film layers on nanosheets. The incorporation of silver film increased the detection limit of this nanosheet for proteins in untreated saliva to 400 pg/mL. The new two-dimensional material MXene can also be used for virus detection, Peng et al. found that the prepared Ta2C MXene material can accurately identify SARS-CoV-2 S by comparing the Raman characteristic peaks of SARS-CoV-2 S protein on Au nanoparticle substrates (Fig. 7d), and the LOD of this system is low quality 5 × 10–9 M [59].
a Schematic diagram of identifying the lysed SARS-CoV-2 by SERS [70]; b Raman spectra of 10–6-10–14 M SARS-CoV-2 S protein on SnS2 microspheres [70]; c Raman spectra of SARS-CoV-2S protein at concentrations ranging from 1.0 × 10–7 to 1.0 × 10–16 M collected on SnS2/Si at 633 nm [71]; d Raman spectra of SARS-CoV-2 S protein on Ta2C NSs and Au NPS substrates [59]; e SERS sensing chip for detection of SARS-CoV-2 RNA in human saliva by SERS spectroscopy [73]
In addition, Achadu et al. innovatively designed a PH-responsive encapsulated nanogel to regulate SERS activity of 2D material substrate [74]. A biosensing platform was developed based on ultra-sensitive immunoassays for Hepatitis E virus (HEV) or Norovirus (NoV) with a two-dimensional h-BN substrate, and the development of nanogels enabled the detection limits of HEV-LPS and NOV-LPS to be 6.5 and 8.2 fg/mL, respectively, with enhancement factors up to 108. Furthermore, the sensor platform can be used not only for highly sensitive detection of viruses, but also for cell or tumor imaging. With the deepening of research, SERS technology has begun to detect clinical samples. Sudarson Sekhar Sinha et al. based on nanostructure GO, can achieve fingerprint diagnosis for drug resistance at 10 CFU/mL level and mosquito-borne flavivirus at 10 PFU/mL level [75]. At the same time, the detection level of Alzheimer's biomarkers by two-dimensional SERS substrates based on nanostructures was as low as 500 fg/mL, which is much higher than the current clinically used technology.
4.1.2 Application of SERS in disease diagnosis
Cancer is a malignant disease that poses a significant threat to human life and health.Identifying it in its early stages accurately is crucial for improving patient survival rates and minimizing unnecessary economic losses. Given that each cancer cell line exhibits unique cellular biomarkers (i.e., genomic and proteomic), distinguishing them from one another is feasible [76]. Consequently, we can employ the distinctive characteristics of these biomarkers to identify cancer cells effectively. However, the accurate identification of cancer cells necessitates the development of novel methods that enable sensitive and selective detection through precise identification of the biomarkers they express. SERS technology is a non-destructive detection method at the single molecule level, boasting high sensitivity and specificity [77]. As such, it offers substantial advantages in the early diagnosis of cancer.
Currently, SERS technology has witnessed significant advancement in the realm of cancer detection. BP has emerged as a valuable component in field effect photoacoustic bioimaging and various other domains. With only three distinct Raman fingerprint peaks occurring below 500 cm−1 [78], BP stands distinctively apart from the typical fingerprint region of biological samples (ranging from 600 to 1800 cm−1), thereby ensuring no interference with biomarker signals. Hence, BP nanosheets prove to be ideal substrate materials for SERS biosensors. Lin et al. compared the SERS results of BP/Ag NS and commercial Au nanoparticles(Fig. 8b), proposed a polarization mapping strategy to identify different tumor exosomes, realize cancer detection and early warning [79]. At the same time, combined with machine learning to accurately identify tumor exosomes, the accuracy rate is as high as 99.17%. The addition of noble metals can not only increase the stability of the structure by forming chemical bonds, but also improve the photoelectric properties and SERS activity of two-dimensional materials. As shown in Fig. 8a [80], Yang et al. used a one-step synthesis method to prepare BP nanosheets loaded with Au nanoparticles. The team used BP-Au nanosheets as nanoagents to conduct in vivo and in vitro experiments for tumor photothermal therapy (PTT). At the same time, the high SERS activity of BP-Au nanosheets was used to clarify the fingerprint characteristics of breast tumor molecules before and after PTT treatment. Ma et al. designed a biosensor based on BP NSs to achieve ultra-sensitive and recyclable detection of ferritin (FER) in human serum (Fig. 8d) [81]. As shown in Fig. 8e, Due to the synergistic effect of molecular enrichment and chemical enhancement, the ultrasensitive detection of 10–4 μg/mL FER can be achieved [81]. Serum samples of 10 clinical patients were analyzed by SERS immunoassay using this method. The results show that compared with the conventional CLIA method, the relative error of this strategy is ± 6.5%.
a Schematic illustration of fabrication of BP–Au NSs and biomedical application [80]; b Raman spectra of A549 exosomes with concentration 1 × 109 particles mL−1 on Ag/BP-NS and commercial gold substrate [79]; c Schematic diagram of quantitative SERS detection of wild-type KRAS ranging from 5 nm to 50 fM [82]; d SERS diagram of FER immune detection in serum; e SERS spectrum of the nonmetallic immunosensor for the detection of clinical serum samples [81]
In addition to, Lin Duo et al. developed a non-destructive, sensitive blood analysis method based on polarized SERS to realize the molecular diagnosis of colorectal cancer [83]. Compared with conventional SERS technology, polarized SERS is more sensitive to differences between patients and normal people. Its accuracy for serum classification is as high as 91.6%. The protocol has the potential to be clinically referenced as an auxiliary screening tool for colorectal cancer. Li et al. proposed a proportional strategy based on SERS technology to achieve quantitative detection of different breast cancer cells [84]. In this method, epidermal growth factor receptor (EGFR) biomarker was selected as the internal standard, and SERS intensity ratio only depended on the expression of the biomarker, effectively excluding the influence of cell concentration, measurement conditions and nanoprobe concentration. Currently, the strategy is a promising method for breast cancer diagnosis and screening, while it can be expanded to detect other cancer cell types. Then, Wu et al. integrated 2D SERS sensors into microfluidic chips to distinguish between mutant and wild-type KRAS genes and various nucleic acids from cancer cells within 40 min (Fig. 8c) [82]. The method is simple to operate and fast to analyze, providing potential insights for the clinical treatment of cancer.
Early diagnosis of diseases is essential for timely diagnosis, treatment and including saving medical resources. Fraser et al. prepared few-layer MoTe2 NSs with high SERS activity by CVD method and used them as the SERS detection substrate for lipid disease marker β-sitosterol (Fig. 9a) [85]. Figure 9b indicates that the LOD of this substrate for the marker β-sitosterol is 10–9 M, while its intensity of SERS peak (1668 cm−1) versus β-sitosterol concentration shows a good linear correlation as shown in Fig. 9c. This is one of the lowest biosensors reported in the SERS detection of sterol lipophilic molecules so far. Satuart et al. developed an Ag NSs biosensor for the in vivo quantification of glucose in animal models. The researchers subcutaneously implanted these Ag NSs into Sprague Dawley rats [86]. Through spectral localization via an optical window, the sensor exhibited high SERS activity and precision, with a RMSEC of 7.46 mg/dL (0.41 mM) and RMSEP of 53.42 mg/dL (2.97 mM). Furthermore, this biosensor offers the advantage of convenient internal standards, the ability to exclude all types of interferents, and enhanced glucose signal detection. This advancement addresses critical limitations that previously hindered the application of SERS technology for glucose determination.
a Synthesis of MoTe2 film and subsequent application of the prepared 2D film to SERS sensing of β-sitosterol schematic diagram [85]; b SERS spectra of sitosterol measured on 2D MoTe2 films (seven layers) after deposition at 10−4, 10−6, 10−8, and 10−9 M concentrations on seven-layered MoTe2 surface [85]; c Dependence of intensity of representative SERS peaks (1668 cm−1) on βsitosterol concentration [85]
The current application of SERS technology for the noninvasive identification of lung cancer predominantly depends on volatile organic compounds (VOCs) as biomarkers. However, the limited Raman scattering of VOCs and their low adsorption capacity on solid matrices significantly constrain the efficacy of SERS technology. Qiao et al. developed a biosensor utilizing a 2D array constructed from RGO. This design was intended to decelerate the flow rate of gaseous biomarkers, thereby enhancing their molecular adsorption [87]. The sensor demonstrated its potential in detecting early and late gastric cancer VOC biomarkers in both simulated and real respiratory samples, achieving a detection limit of 10 ppb. This underscores its significant potential for in vitro diagnosis of early-stage lung cancer.
Two dimensional materials are characterized as planar substances, each with a thickness equivalent to a single or multiple atomic layers. These materials offer an abundance of surface active sites and present a surface that is readily functionalized [88]. At present, they have a wide range of applications in fields such as biosensing. With the increasing number of reported highly SERS-active materials and means to enhance SERS performance, SERS technology is expected to be further developed into practical applications in biomedicine [89].
4.2 The application of SERS technology in food safety
The application of SERS technology in the domain of food safety has garnered significant attention. This cutting-edge analytical technique provides unparalleled sensitivity and specificity, rendering it indispensable for detecting and quantifying trace amounts of contaminants in food products. By harnessing spectroscopic principles, SERS enables the identification of specific molecules or compounds present at concentrations as low as parts per billion (ppb). Such capabilities render it particularly suitable for monitoring food supply chains, ensuring the integrity of raw materials, processing equipment, and finished products. Moreover, SERS finds utility in evaluating the safety and quality of food products by analyzing their composition, detecting potential allergens or toxins, and assessing the effectiveness of additives or preservatives. As the global demand for safe and nutritious food continues to escalate, the role of SERS in augmenting food safety practices is poised to become increasingly indispensable [90].
Malachite green, a type of fish drug that is explicitly prohibited by the state due to its potential carcinogenic and teratogenic risks to humans, can be detected with a 2D BCN designed by Liang et al. The limit of detection for malachite green is remarkably low, at 10–8 M [53]. Sun et al. successfully combined alumina nanorods with Ag nanoparticles to trace and detect unhealthy chemical residues in oil and fat (Fig. 10a) [91]. In contrast, while Radu et al. used SERS technology to concurrently detect two B vitamins (riboflavin, vitamin B2 and cyanocobalamin, vitamin B12), the limit of detection was surprisingly low at 70 nM (Fig. 10d,e) [92]. This method not only offers superior analytical sensitivity compared to traditional HPLC methods, but also reduces both detection time and associated costs. Cui et al. combined hydrothermal reaction and magnetron sputtering technology to design a nanosheet structure of ZnO doped Ag [93]. The detection limit of malachite green can be as low as 10–12 M, with an enhancement factor of 2.5 × 1010. In addition, the SERS substrate can be used for sensitive detection of low concentration molecules related to food safety. Wang et al. successfully employed SERS technology to facilitate swift and efficient micro-area direct sampling of intricate surfaces, including those found in cucumbers, apples, and grapes. Concurrently, they utilized this technology to directly detect several pesticide residues within real samples, namely Tebuconazole (TMTD), Methyl Parathion (MPT), and Malachite Green (MG). This process is illustrated in Fig. 10b [93]. Under optimal conditions, the sensitivity of TMTD to apple peel was measured at 1.6 ng/cm2 (S/N = 3), with a high correlation coefficient (R) of 0.99. Bisphenol A (BPA) is an endocrine-disrupting chemical that is prevalent in both environmental and food containers. Exposure to even low levels of BPA can disrupt the human endocrine system, leading to cardiovascular diseases and other health issues. To achieve trace detection of BPA, Quan et al. utilized MoS2 and ZnO nanoparticles to create a heterogeneous structure of composite materials for SERS substrates [94]. This method allows for the detection of BPA contaminants with a limit of detection as low as 1 nm. Chen et al. employed MoS2 as a SERS substrate to achieve ultra-sensitive detection of low concentration melamine solutions (Fig. 10c), thereby providing technical support for food safety [95]. Sun et al. employed plasma-enhanced CVD technology to fabricate graphene wall (G-wall) nanostructures. This structure exhibited high SERS activity while effectively eliminating fluorescence noise, indicating significant potential for practical applications [96].
4.3 The application of SERS technology in environmental monitoring
The utilization of SERS technology in environmental monitoring has garnered significant attention in recent years. This advanced technique offers a powerful tool for detecting and quantifying various environmental parameters, making it particularly valuable in fields such as ecology, atmospheric science, and water research [97]. By leveraging the unique properties of selenium-rich materials, SERS can detect trace amounts of contaminants with unparalleled sensitivity, making it an indispensable instrument for both routine environmental monitoring and emergency response scenarios. Furthermore, its ability to simultaneously analyze multiple samples simultaneously enhances its applicability in complex environments. As the field continues to evolve, advancements in imaging and detection systems are pushing the effects of what SERS can achieve, making it an increasingly vital component of modern environmental research [98].
Pb2+ is one of the most toxic heavy metal ions present in water. It can easily accumulate in biological species, and even low concentrations can cause significant damage to the kidneys, immune system, and cognitive development. The porous nanostructure offers a high surface area that promotes increased adsorption of heavy metal ions. Additionally, the large number of defects provides more active binding sites, while the abundant surface states facilitate charge transfer, thereby enhancing the performance of SERS. Taking advantage of these properties, Liu et al. successfully designed porous ZnO nanosheets [99]. Due to their porosity and surface defect content (specific surface area of 98.8 m2 g−1), this material can provide an extremely low detection limit for 4-Methylbenzylamine (4-MBA), which has been utilized in river water pollution detection to date. Building on 2D ZnO materials, Zhang et al. developed a novel ZnO Nanofibers SERS biosensor [100], significantly improving the SERS performance of original ZnO nanoparticles. Currently, this biosensor has been used in the detection of the heavy metal ion Pb2+, maintaining a linear relationship between 10 pM ~ 100 μM. Similarly, conducting SERS detection for Pb2+ is also imminent. Self-assembled Au nanostar dimer SERS substrate shown in Fig. 11a can reduce the detection limit to 0.8 pg/mL due to the presence of strong electromagnetic enhancement fields contributed by hot spot effect. As shown in Fig. 11b, Ag nanorod array substrate modified with thymine T-Cy5 dye is also enhanced by hot spot effect, and Hg2+ ions are captured into the SERS enhanced area through thymine, with a low detection limit as low as 0.16 pM [97]. The above three kinds of SERS substrates are all direct SERS detection of Hg2+ ions in water environment.
CuPc is a frequently utilized organic compound in industry, and as a result, the discharge of factory wastewater frequently results in an elevated CuPc concentration in river water. Consequently, it is crucial to perform sensitive CuPc detection. Yin et al. have successfully synthesized 1T-MoSe2 and 1T-MoS2 exhibiting SERS activity via phase engineering [104], achieving a detection limit for pollutant CuPc as low as 10–9 M. This significant achievement paves the way for future research into phase engineering to enhance the SERS performance of materials. Furthermore, it is noteworthy that the orientation of substrate molecules can significantly influence the SERS performance of the substrate itself. Lin et al. successfully implemented SERS detection of CuPc utilizing a 2D layered material ReSe2 [105]. Upon interaction with the ReSe2 substrate, randomly oriented CuPc molecules initiated anisotropically correlated scattering. This discovery offers novel perspectives on charge transfer mechanisms in CM. Liu et al. significantly improved the EF ratio by over 40 times on a WSe2 monolayer with an atomic ratio of 1.96 [106], compared to the original WSe2 monolayer. This enhancement was achieved by adjusting the atomic ratio of WSe2. As a result, there was a ninefold increase in the exciton number and a 9.4 fold increase in the charge transfer current between the probe molecule and WSe2. This led to the successful quantitative detection of CuPc.
As shown in Fig. 11 (a), Xie et al. devised a SERS substrate composed of Ti3C2Tx MXene nanosheets and Au nanoparticles composites for the purpose of trace detection of organic pollutants [101]. The detection limits of this SERS substrate for p-cymene and diquat, two environmental pollutants, were respectively measured at 1 × 10−10 M and 1 × 10−8 M (Fig. 11d,e). These levels are considerably lower than those regulated by the US Environmental Protection Agency. Diquat (DQ) is among the most extensively used herbicides globally, following the ban of paraquat. The residues of DQ in food and water resources pose significant threats to human health. To facilitate their detection, Jiang et al. employed a combination of MoS2 NSs and Au nanoparticles as a SERS substrate for the direct SERS detection of DQ from complex compounds This method eliminates the need for pretreatment, with its SERS test results depicted in Fig. 11c [103]. It boasts a remarkable limit of detection as low as 10–15 M. Zhao et al. created a glass fiber membrane by integrating MoS2 nanosheets (Fig. 11b) and Au nanoparticles on glass fibers [102]. The SERS substrate demonstrated the ability to detect CV and TB molecules in water with remarkable sensitivity, exhibiting detection limits of up to 10–7 M and 10–8 M respectively.
Two-dimensional materials are among the most extensively studied materials currently, attracting researchers due to their unique layered structure that is different from three-dimensional bulk materials and the advantages such as quantum confinement effect. Transition metal dichalcogenides, MXene, derivatives of graphitic structures, and emerging black phosphorus and other two-dimensional materials have been reported to possess excellent SERS activity, which lays a foundation for the development of ultrasensitive SERS substrates and the advancement of SERS technology. As shown in Table 1, many two-dimensional materials exhibit comparable SERS activities with noble metals. However, materials exhibiting SERS activity have mostly remained confined to laboratory research, yet to bridge the gap towards clinical applications. Consequently, there is an ongoing need to focus research efforts on the development and enhancement of materials with both high SERS activity and exceptional biocompatibility, aligning with the practical demands of clinical applications.
5 Summary and prospect
Two-dimensional materials, due to their unique properties, have been a topic of extensive research. This paper presents a comprehensive review of the latest advancements in two-dimensional materials within the field of SERS. We begin by discussing the origins of SERS technology, followed by an introduction to its development process and the widely accepted enhancement mechanism. The preparation methods of two-dimensional materials are then explored, which include chemical vapor deposition, sintering, and hydrothermal method. Through doping, shaping and controlling the morphology of two-dimensional materials, detection limits as low as 10–13 M can be achieved when these materials are used as SERS substrates. Current research on SERS of two-dimensional materials primarily focuses on graphite-like structures, transition metal sulfides, MXene materials, black phosphorus and more. Specifically, transition metal sulfides and MXene materials possess semi-metallic properties, which enhance the sensitivity of SERS technology. In conclusion, the utilization of SERS technology in biosensing, food safety, and environmental detection is comprehensively reviewed. Given the superior biocompatibility and selective enhancement capabilities of two-dimensional semiconductor materials, they are particularly apt for clinical applications via SERS. This paper provides an in-depth overview of SERS technology in biosensing, encompassing virus detection, cancer detection, drug detection, and contemporary biological imaging. To date, numerous esteemed scholars have presented innovative research findings and ideas to further this field of study. A significant number of these researchers have conducted SERS experiments using clinical samples, contributing valuable practical experience for the implementation of SERS technology. As a super-sensitive detection technology for fingerprint recognition, SERS has high applicability in the field of biosensing and is increasingly gaining traction in real-world applications. The maturity of technologies such as microfluidic chips, LFA platforms, and machine learning have also aided in the clinical.
References
Fleischmann M, Hendra PJ (1974) McQuillan AJ (1973) Raman spectra of pyridine adsorbed at a silver electrode. Chem Phys Lett 26:163–166
Jeanmaire DL, Van Duyne RP (1977) Surface Raman spectroelectrochemistry: Part I. Heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode. J Electroanal Chem Interfacial Electrochem 84:1–20
Kohn W, Sham L (1965) Self-consistent equations including exchange and correlation effects. Phys Rev 140:1133
Mayer KM, Hafner JH (2011) Localized surface plasmon resonance sensors. Chem Rev 111:3828–3857
Verbruggen SW, Keulemans M, Martens JA et al (2013) Predicting the surface plasmon resonance wavelength of gold-silver alloy nanoparticles. J Phys Chem C 117:19142–19145
Lee K-S, El-Sayed MA (2006) Gold and silver nanoparticles in sensing and imaging: sensitivity of plasmon response to size, shape, and metal composition. J Phys Chem B 110:19220–19225
Yang Y, Li Z-Y, Yamaguchi K et al (2012) Controlled fabrication of silver nanoneedles array for SERS and their application in rapid detection of narcotics. Nanoscale 4:2663
Wiley BJ, Im SH, Li Z-Y et al (2006) Maneuvering the surface plasmon resonance of silver nanostructures through shape-controlled synthesis. J Phys Chem B 110:15666–15675
Link S, Wang ZL, El-Sayed MA (1999) Alloy formation of gold−silver nanoparticles and the dependence of the plasmon absorption on their composition. J Phys Chem B 103:3529–3533
Loo B (1982) Observation of the surface enhanced Raman-scattering effect from the semiconductor - electrolyte interface. J Electroanal Chem 136:209–213
Yamada H, Yamamoto Y (1983) Surface enhanced Raman-scattering (SERS) of chemisorbed species on various kinds of metals and semiconductors. Surf Sci 134:71–90
Potts J, Merlin R, Partin D (1983) Roughness-induced Raman-scattering from surface carbon on Pbte. Phys Rev B 27:3905–3908
Li M, Gao Y, Fan X et al (2021) Origin of layer-dependent SERS tunability in 2D transition metal dichalcogenides. Nanoscale Horiz 6:186–191
You Q, Li Z, Li Y et al (2022) Resonance photoluminescence enhancement of monolayer MoS 2 via a plasmonic nanowire dimer optical antenna. ACS Appl Mater Interfaces 14:23756–23764
Li J, Xu X, Huang B et al (2021) Light-induced in situ formation of a nonmetallic plasmonic MoS2/MoO3–x heterostructure with efficient charge transfer for CO2 reduction and SERS detection. ACS Appl Mater Interfaces 13:10047–10053
Wen C, Liao Q, Cui Z et al (2023) Exfoliation behavior and superior photothermal conversion performance of MXenes beyond Ti3C2Tx. Chem Eng J 472:144921
Han M, Shuck CE, Rakhmanov R et al (2020) Beyond Ti3C2Tx: MXenes for electromagnetic interference shielding. ACS Nano 14:5008–5016
Li Z (2018) Mesoscopic and microscopic strategies for engineering plasmon-enhanced Raman scattering. Adv Opt Mater 6:1701097
Moskovits M (1985) Surface-enhanced spectroscopy. Rev Mod Phys 57:783–826
Lombardi JR, Birke RL (2009) A unified view of surface-enhanced Raman scattering. Acc Chem Res 42:734–742
Albrecht AC (1961) On the theory of Raman intensities. J Chem Phys 34:1476–1484
Lombardi JR, Birke RL (2008) A unified approach to surface-enhanced Raman spectroscopy. J Phys Chem C 112:5605–5617
Alessandri I, Lombardi JR (2016) Enhanced Raman scattering with dielectrics. Chem Rev 116:14921–14981
Jiao L, Hu M, Peng Y et al (2016) Electronic, elastic, and optical properties of monolayer BC2N. J Solid State Chem 244:120–128
Lin J, Zheng J, Wu A (2020) An efficient strategy for circulating tumor cell detection: surface-enhanced Raman spectroscopy. J Mater Chem B 8:3316–3326
Jiang D, Yang L, Yuan H et al (2022) Saturated hydrogen regulated ti coordination of metallic tih2/ti electrode via in-situ electrochemical hydrogenation for enhanced hydrogen evolution reaction. Nano Energy 93:106892
Yue S, Ye W, Xu Z (2019) SERS monitoring of the Fenton degradation reaction based on microfluidic droplets and alginate microparticles. Analyst 144:5882–5889
Kneipp K, Wang Y, Kneipp H et al (1997) Single molecule detection using surface-enhanced Raman scattering (SERS). Phys Rev Lett 78:1667–1670
Nie S, Emory SR (1997) Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science 275:1102–1106
Qiu C, Bao Y, Netzer NL et al (2013) Structure evolution and SERS activation of cuprous oxide microcrystals via chemical etching. J Mater Chem A 1:8790–8797
Lin C, Li Y, Peng Y et al (2023) Recent development of surface-enhanced Raman scattering for biosensing. J Nanobiotechnology 21:149
Luther JM, Jain PK, Ewers T et al (2011) Localized surface plasmon resonances arising from free carriers in doped quantum dots. Nat Mater 10:361–366
Campion A, Kambhampati P (1998) Surface-enhanced Raman scattering. Chem Soc Rev 27:241–250
Tao L, Chen K, Chen Z et al (2018) 1t’transition metal telluride atomic layers for plasmon-free SERS at femtomolar levels. J Am Chem Soc 140:8696–8704
Shen Y, Han Y, Zhan R et al (2020) Pyramid-shaped single-crystalline nanostructure of molybdenum with excellent mechanical, electrical, and optical properties. ACS Appl Mater Interfaces 12:24218–24230
Ji W, Li L, Guan J et al (2021) Hollow multi-shelled V2O5 microstructures integrating multiple synergistic resonances for enhanced semiconductor SERS. Adv Opt Mater 9:2101866
Ozturk MS, Chen C-W, Ji R et al (2016) Mesoscopic fluorescence molecular tomography for evaluating engineered tissues. Ann Biomed Eng 44:667–679
Milekhin AG, Sveshnikova LL, Duda TA et al (2016) Surface-enhanced Raman spectroscopy of semiconductor nanostructures. Phys E-Low-Dimens Syst Nanostructures 75:210–222
Yang LL, Peng YS, Yang Y et al (2019) A novel ultra-sensitive semiconductor SERS substrate boosted by the coupled resonance effect. Adv Sci 6:1900310
Yang LL, Yang Y, Lombardi JR et al (2020) Charge transfer enhancement in the surface-enhanced Raman scattering of Ta2O5 superstructures. Appl Surf Sci 520:146325
Wang Y, Wang L, Zhang X et al (2021) Two-dimensional nanomaterials with engineered bandgap: Synthesis, properties, applications. Nano Today 37:101059
Zheng Q, Suzuki K, Hiraga Y et al (2023) Understanding the reaction behavior of alanine under hydrothermal conditions through a network model. Chem Eng J 468:143588
Li F, Li Y, Zhao Y et al (2023) Synthesis of graphene nanomesh with symmetrical fractal patterns via hydrogen-free chemical vapor deposition. Nanotechnology 34:045601
Suzuki S, Yoshimura M (2017) Chemical stability of graphene coated silver substrates for surface-enhanced Raman scattering. Sci Rep 7:14851
Nalini S, Thomas S, Anju KS et al (2023) Chemical vapour deposited graphene-mediated enhanced SERS performance in silver nanostructures. Mater Sci Technol 39:933–940
Ayhan ME, Emeller N (2023) Surface enhancement Raman spectroscopy of CVD graphene and graphene oxide. Fullerenes, nanotubes and carbon nanostructures. pp 1–4
Zhao S, Wang H, Niu L et al (2021) 2D GaN for highly reproducible surface enhanced Raman scattering. Small 17:2103442
Song G, Gong W, Cong S et al (2021) Ultrathin two-dimensional nanostructures: surface defects for morphology-driven enhanced semiconductor SERS. Angew Chem Int Ed 60:5505–5511
Lv Q, Wu X, Tan J et al (2021) Ultrasensitive molecular sensing of few-layer niobium diselenide. J Mater Chem A 9:2725–2733
Iravani S, Varma RS (2023) MXene-based hybrid structures for ultraviolet photodetectors. Matter 6:2525–2527
Li X (2023) Customizing MXenes. Matter 6:2519–2522
Yang LL, Peng YS, Yang Y et al (2018) Green and sensitive flexible semiconductor SERS substrates: hydrogenated black TiO2 nanowires. ACS Appl Nano Mater 1:4516–4527
Liang C, Lu Z-A, Zheng M et al (2022) Band structure engineering within two-dimensional borocarbonitride nanosheets for surface-enhanced Raman scattering. Nano Lett 22:6590–6598
Li X, Guo S, Su J et al (2020) Efficient Raman enhancement in molybdenum disulfide by tuning the interlayer spacing. ACS Appl Mater Interfaces 12:28474–28483
Zhang Y, Yang T, Li J et al (2023) Construction of Ru, O Co-doping MoS2 for hydrogen evolution reaction electrocatalyst and surface-enhanced Raman scattering substrate: high-performance, recyclable, and durability improvement. Adv Funct Mater 33:2210939
Jha G, Soren S, Mehta KD (2023) Assessment of the internal chemistry nexus of coke and biomass-based sinters. Sādhanā 48:217
Wang X, Shi W, Jin Z et al (2017) Remarkable SERS activity observed from amorphous ZnO nanocages. Angew Chem Int Ed 56:9851–9855
Wang X, Shi W, Wang S et al (2019) Two-dimensional amorphous TiO2 nanosheets enabling high-efficiency photoinduced charge transfer for excellent SERS activity. J Am Chem Soc 141:5856–5862
Peng YS, Lin CL, Long L et al (2021) Charge-transfer resonance and electromagnetic enhancement synergistically enabling MXenes with excellent SERS sensitivity for SARS-CoV-2 S protein detection. Nano-Micro Lett 13:52
He Z, Rong T, Li Y et al (2022) Two-dimensional TiVC solid-solution MXene as surface-enhanced Raman scattering substrate. ACS Nano 16:4072–4083
Naguib M, Kurtoglu M, Presser V et al (2011) Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv Mater 23:4248–4253
Xu X, Guo T, Lanza M et al (2023) Status and prospects of MXene-based nanoelectronic devices. Matter 6:800–837
Lan L, Fan X, Yu S et al (2022) Flexible two-dimensional vanadium carbide MXene-based membranes with ultra-rapid molecular enrichment for surface-enhanced Raman scattering. ACS Appl Mater Interfaces 14:40427–40436
Luo W, Xiong W, Han Y et al (2022) Application of two-dimensional layered materials in surface-enhanced Raman spectroscopy (SERS). Phys Chem Chem Phys 24:26398–26412
Carter LJ, Garner LV, Smoot JW et al (2020) Assay techniques and test development for COVID-19 diagnosis. ACS Cent Sci 6:591–605
Okamoto Y, Katsuyama H (1996) Preparation, catalysis, and characterization of highly dispersed molybdenum sulfide catalysts supported on a NaY zeolite. Ind Eng Chem Res 35:1834–1844
Zhang M, Li X, Pan J et al (2021) Ultrasensitive detection of SARS-CoV-2 spike protein in untreated saliva using SERS-based biosensor. Biosens Bioelectron 190:113421
Li YY, Peng YS, Lin CL et al (2023) Nanomaterials and biosensing technology for novel coronavirus detection. J Inorg Mater 38(01):3–31
Yang Y, Peng Y, Lin C et al (2021) Human ACE2-functionalized gold “Virus-Trap” nanostructures for accurate capture of SARS-CoV-2 and single-virus SERS detection. Nano-Micro Lett 13:109
Peng Y, Lin C, Li Y et al (2022) Identifying infectiousness of SARS-CoV-2 by ultra-sensitive SnS2 SERS biosensors with capillary effect. Matter 5:694–709
Li F, Mu X, Tang X et al (2023) Semiconductor SERS on colourful substrates with Fabry-Pérot cavities. Angew Chem 135:e202218055
Lin X, Weng Y, Liu Y et al (2024) Ratiometric SERS sensing chip for high precision and ultra-sensitive detection of SARS-CoV-2 RNA in human saliva. Sens Actuators B Chem 399:134803
Kaladharan K, Chen KH, Chen PH et al (2023) Dual-clamped one-pot SERS-based biosensors for rapid and sensitive detection of SARS-CoV-2 using portable Raman spectrometer. Sens Actuators B Chem 393:134172
Achadu OJ, Abe F, Li T-C et al (2021) Molybdenum trioxide quantum dot-encapsulated nanogels for virus detection by surface-enhanced Raman scattering on a 2D substrate. ACS Appl Mater Interfaces 13:27836–27844
Sinha SS, Jones S, Pramanik A et al (2016) Nanoarchitecture based SERS for biomolecular fingerprinting and label-free disease markers diagnosis. Acc Chem Res 49:2725–2735
Lin T, Song YL, Kuang P et al (2021) Nanostructure-based surface-enhanced Raman scattering for diagnosis of cancer. Nanomedicine 16:2389–2406
Wang D, Yi P, Wang L et al (2019) Revisiting the growth of black phosphorus in Sn-I assisted reactions. Front Chem 7:21
Mousavi SM, Hashemi SA, Rahmanian V et al (2022) Highly sensitive flexible SERS-based sensing platform for detection of COVID-19. Biosensors 12:466
Lin CL, Liang SS, Peng YS et al (2022) Visualized SERS imaging of single molecule by Ag/Black phosphorus nanosheets. Nano-Micro Lett 14:75
Yang G, Liu Z, Li Y et al (2017) Facile synthesis of black phosphorus–Au nanocomposites for enhanced photothermal cancer therapy and surface-enhanced Raman scattering analysis. Biomater Sci 5:2048–2055
Ma J, Xue D, Xu T et al (2023) Nonmetallic SERS-based biosensor for ultrasensitive and reproducible immunoassay of ferritin mediated by magnetic molybdenum disulfide nanoflowers and black phosphorus nanosheets. Colloids Surf B Biointerfaces 227:113338
Wu L, Garrido-Maestu A, Guerreiro JRL et al (2019) Amplification-free SERS analysis of DNA mutation in cancer cells with single-base sensitivity. Nanoscale 11:7781–7789
Lin D, Huang H, Qiu S et al (2016) Diagnostic potential of polarized surface enhanced Raman spectroscopy technology for colorectal cancer detection. Opt Express 24:2222
Li L, Liao M, Chen Y et al (2019) Surface-enhanced Raman spectroscopy (SERS) nanoprobes for ratiometric detection of cancer cells. J Mater Chem B 7:815–822
Fraser JP, Postnikov P, Miliutina E et al (2020) Application of a 2D molybdenum telluride in SERS detection of biorelevant molecules. ACS Appl Mater Interfaces 12:47774–47783
Stuart DA, Yuen JM, Shah N et al (2006) In vivo glucose measurement by surface-enhanced Raman spectroscopy. Anal Chem 78:7211–7215
Qiao X, Su B, Liu C et al (2018) Selective surface enhanced Raman scattering for quantitative detection of lung cancer biomarkers in Superparticle@ MOF Structure. Adv Mater 30:1702275
Guan H, Li W, Han J et al (2021) General molten-salt route to three-dimensional porous transition metal nitrides as sensitive and stable Raman substrates. Nat Commun 12:1376
Zhang L, Yi W, Li J et al (2023) Surfactant-free interfacial growth of graphdiyne hollow microspheres and the mechanistic origin of their SERS activity. Nat Commun 14:6318
Liao W, Lu X (2016) Determination of chemical hazards in foods using surface-enhanced Raman spectroscopy coupled with advanced separation techniques. Trends Food Sci Technol 54:103–113
Sun K, Huang Q (2016) Detection of chemical residues in food oil via surface-enhanced Raman spectroscopy. In: Kim MS, Chao K, Chin BA (eds). p. 98640B. https://doi.org/10.1117/12.2229812
Radu AI, Kuellmer M, Giese B et al (2016) Surface-enhanced Raman spectroscopy (SERS) in food analytics: Detection of vitamins B2 and B12 in cereals. Talanta 160:289–297
Cui S, Dai Z, Tian Q et al (2016) Wetting properties and SERS applications of ZnO/Ag nanowire arrays patterned by a screen printing method. J Mater Chem C 4:6371–6379
You H, Mu Z, Zhao M et al (2019) Voltammetric aptasensor for sulfadimethoxine using a nanohybrid composed of multifunctional fullerene, reduced graphene oxide and Pt@ Au nanoparticles, and based on direct electron transfer to the active site of glucose oxidase. Microchim Acta 186:1
Chen Y, Liu H, Li X et al (2021) Development of RGO@ MoS2@ Ag ternary nanocomposites with tunable geometry structure for recyclable SERS detection. Sens Actuators B Chem 339:129856
Sun T, Gu F, Pu L et al (2017) In situ fabrication of graphene nanowalls as active surface-enhanced Raman scattering substrate. Mater Express 7:398–404
Kamal S, Yang TC-K (2022) Silver enriched silver phosphate microcubes as an efficient recyclable SERS substrate for the detection of heavy metal ions. J Colloid Interface Sci 605:173–181
Wang P, Zhou Y, Zhou Y et al (2017) In-situ growth of raspberry-like silver composites for Raman detection of acrylamide. Sens Actuators B Chem 243:856–862
Liu Q, Jiang L, Guo L (2014) Precursor-directed self-assembly of porous ZnO nanosheets as high-performance surface-enhanced Raman scattering substrate. Small 10:48–51
Zhang H, Huang S, Yang X et al (2021) A SERS biosensor constructed by calcined ZnO substrate with high-efficiency charge transfer for sensitive detection of Pb2+. Sens Actuators B Chem 343:130142
Xie H, Li P, Shao J et al (2019) Electrostatic self-assembly of Ti3C2Tx MXene and gold nanorods as an efficient surface-enhanced Raman scattering platform for reliable and high-sensitivity determination of organic pollutants. ACS Sens 4:2303–2310
Zhao Y, Pan X, Zhang L et al (2017) Dense AuNP/MoS2 hybrid fabrication on fiber membranes for molecule separation and SERS detection. RSC Adv 7:36516–36524
Jiang QY, Li D, Liu Y et al (2021) Recyclable and green AuBPs@ MoS2@ tinfoil box for high throughput SERS tracking of diquat in complex compounds. Sens Actuators B Chem 344:130290
Yin Y, Miao P, Zhang Y et al (2017) Significantly increased Raman enhancement on MoX 2(X = S, Se) monolayers upon Phase Transition. Adv Funct Mater 27:1606694
Lin J, Liang L, Ling X et al (2015) Enhanced Raman scattering on in-plane anisotropic layered materials. J Am Chem Soc 137:15511–15517
Liu Y, Gao Z, Chen M et al (2018) Enhanced Raman scattering of CuPc films on imperfect WSe2 monolayer correlated to exciton and charge-transfer resonances. Adv Funct Mater 28:1805710
Acknowledgements
Not applicable.
Funding
The authors gratefully acknowledge the finical support of the National Key Research and Development Program (Grant No. 2022YFE0110100), Shanghai Sailing Program (No. 23YF1454600).
Author information
Authors and Affiliations
Contributions
YY and YSP proposed the idea and supervised the whole work. WDZ YSP and CLL wrote the main manuscript text. WDZ MMX and SZ prepared Fig. 2. CLL and SZ are responsible for the investigation of existing 2D materials. WDZ and MMX are responsible for the investigation of SERS activity of 2D materials. WDZ is responsible for writing the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
Yong Yang is a member of the editorial board of this journal. He was not involved in the editorial review or the decision to publish this article. All authors declare that there are no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, W., Peng, Y., Lin, C. et al. Research progress and application of two-dimensional materials for surface-enhanced Raman scattering. Surf. Sci. Tech. 2, 14 (2024). https://doi.org/10.1007/s44251-024-00042-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44251-024-00042-9