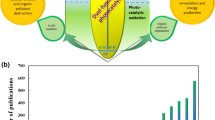
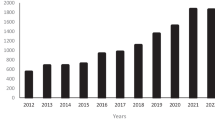
Abstract
With the development of society, human beings are facing environmental problems and an energy crisis worldwide. In this context, photocatalysis and electrocatalysis represent promising technologies to help solving these issues. Up to now, most of the catalysts intended for these usages are prepared via time-consuming wet-chemical approaches, e.g. hydrothermal or sol–gel methods. Moreover, these techniques produce powdery catalysts which need not only a post-filtration step, but also a shaping by a binder for their final applications, which makes the manufacturing cumbersome. Thermal spraying is currently a well-established deposition technique that is capable of elaborating a wide series of functional coatings based on all classes of materials (metals, polymers, ceramics) and featuring tunable compositions as well as micro- and nanostructures. In particular, thermal involving liquid feedstock, and more specifically precursor solutions or suspensions, have allowed generating a wide range of coating thicknesses, from the ten-micron to the submicron/nano scale, increasing thereby the possibilities for enhancing catalytic performances. The present review sets out marking researches relating to the preparation and testing of (i) some photocatalytic coatings intended for the degradation of aqueous organic pollutants and (ii) electrocatalytic coatings investigated as potential energy storage devices. This paper will not only deal with the preparation of catalysts via different thermal spray processes, but also will cover the adjustment of phase compositions and microstructures that are rendered possible when using liquid feedstock thermal spray techniques with the prospect of optimizing the catalytic performances.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The progress realized in the field of thermal spraying are paving the way to new applications which were until now reserved for traditional methods. In this regard, it is worth reporting some recent results obtained by thermally sprayed coatings in the fields of pollutant degradation and energy storage. The first field regards the photocatalytic degradation (PCD) of organic pollutants that are wasted by e.g. the textile industry worldwide [1]. As early as in the 90’s, pioneering works have unvailed the photocatalytic degradation (PCD) pathway as a potential water purification technique [2]. As a result, the metal oxide semiconductors (MOS) used in this type of processing, namely TiO2 and ZnO, became popular photocatalysts for the removal of organic pollutants [3]. The second topic regards new devices that are experiencing rapids development in the storage of electricity. In this area, there is a multiplicity of existing electrochemical systems; amog them, Li-ion batteries and supercapacitors are the most investigated devices by researchers. For example, the newly developed supercapacitors possess outstanding advantages due to their high power density and safety level, fast charge and discharge cycles, and long lifetime (> 10,000 cycles) [4, 5].
The optimization of the preparation of photocatalysts and electrocatalysts resorting to conventional sysntesis methods is a very active field of research [6,7,8,9,10]. Taking photodegradation catalysts as an example, various conventional techniques, e.g. hydrothermal, solvothermal, so-gel and co-precipitation, enable adjusting to some extent the morphologies and phase compositions to optimize performances [6]. Regarding more specifically the preparation of electrodes for energy storage, conventional syntheses include also chemical bath deposition, sol–gel, electrophoretic deposition, spin-coating technology [8,9,10]. However, these methods involve multi-step processes, long preparation times, rather low yields, making them uneasy to industrialize. In addition, these conventional routes produce electrode materials in the form of powders, which requires preparation of slurry, adding organic binders and screen to obtain exploitable electrode coatings [11,12,13,14,15]. The incorporation of such binders in supercapacitor and Li-ion batteries, and their progressive ageing create additional issues in terms of activity and stability. The losses of nanoparticles incurred in these processes are also damageable [16,17,18] as well as the necessary use of surfactants or templates, such as hexamethylenetetramine (HMT) or cetyltrimethylammonium bromide (CTAB) [19, 20]. In contrast, thermal spraying is capable of directly depositing photocatalytic and electrocatalytic coatings without any binder.
In view of the drawbacks of the conventional synthesis routes, it is of high interest to develop “greener” routes for the preparation of photocatalysts while (i) avoiding tedious processes and the consumption/wastage of noxious chemicals and (ii) allowing faster and cost-effective production.
This is where thermal spraying can bring significant advantages owing to its simplicity and straightforwardness that enables the direct formation of films rather than noxious nanopowders, a key asset for the industrialization phase. Indeed, thermal spraying offers a rich set of technologies which deliver a large variety of coatings for the industry and have proved their high efficiency and versatility [21] in a large number of applications. Up to now, the thermal spraying processes are most often used to produce protective coatings and the works devoted to photocatalytic and electrocatalytic applications are rather scarce in the literature. Yet thermal spraying is capable to directly deposit binder-free photocatalytic and electrocatalytic coatings. More specifically, the relatively novel “solution precursor thermal spray” (SPTS) and “suspension thermal spray” (STS) processes which use aqueous solutions as feedstocks, offer flexible possibilities for depositing nanostructured films. Indeed, as shown later in this paper, nanostructuration is a key factor for high photocatalytic and electrocatalytic performances [21,22,23]. It is therefore timely to explore the capabilities of these techniques as potential options for the depollution and the storage of green energies.
The present review of the state-of-the-art works carried out in these fields is focused on presenting successively (i) the deposition principles of different thermal spraying techniques, (ii) some major developments and applications achieved in the fields of photocatalytic and electrocatalytic coatings.
2 Thermal spray processes for photocatalytic and electrocatalytic coatings
The concept of thermal spraying was created by Max Ulrich Schoop in 1910 in Zurich. Thermal spray processes comprise a family of coating technologies in which a stream of finely divided metals are deposited on a substrate, in a molten or semi-molten form, to form a continuous coating [21]. The thermal spray torch heats and melts the feedstock and accelerates the resulting particles towards the substrates, forming morphologies known as “splats” or “lamellar-structures” [21, 24, 25]. A schematic of this process is shown in Fig. 1. The final coatings can be built-up by depositing the feedstock material after a certain number of passes. The heated and accelerated droplets or particles are able to (i) plastically deform on impact and rapidly cool into thin lamellae; (ii) adhere to the surface, and (iii) overlap and interlock into a consolidated layer during the rapid solidification process [24]. Depending on the source of energy, thermal-spray processes can be categorized as combustion, electric arc and kinetic, as shown in Fig. 1 [24, 26]. Due to the roughness of substrate surface by sand-blasting and the rapid cooling procedure of each splat, the thermal sprayed, the basic bonding mechanism of thermal sprayed coatings is mechanical interlocking, where the bonding strength between splats can be improved by increasing temperature or particle velocities during particle impact on the substrates [27]. In the kinetic thermal spray (also called “cold spray”), the coatings are formed by way of the plastic deformation of ductile metallic or alloyed powders, which differs from combustion spraying or electrical-discharge plasma spraying [21]. Owing to the strong impact between particle and substrate, the kinetic energy of powder was converted into heat, and severe plastic deformation happend upon impact with the substrate. As a result, the bonding mechanism of cold sprayed coatings is combination of mechanical interlocking and metallurgical bonding [27]. In fact, the conventional low-pressure or high-pressure cold spraying is not suitable for depositing ceramic materials that are materials of choice in photocatalytic and electrocatalytic applications. Therefore, the following presentation will concentrate on the combustion spray and electrical-discharge thermal spray routes.
Schematic of the thermal spray process [28]
2.1 Combustion spraying
Flame spraying is the oldest thermal-spray technology, characterized by low capital investment, high deposition rates and efficiencies, and relative ease of operation [29].
The high velocity oxy-fuel spray (HVOF) process is a new member of the family of combustion spraying techniques, which employs combustion energy from a gas or liquid fuel to heat the injected powders. A Laval-type nozzle is used to achieve high gas velocity (up to 2,000 m/s). The temperature of flame jet is in the range of 2500 °C to 3200 °C, relying on the type of fuel, the ratio of fuel gas/ oxygen as well as the gas pressure. Owing to the use of air instead of oxygen, high velocity air–fuel spray (HVAF) generates lower gas temperature and higher gas velocities than HVOF [21, 29]. The detonation gun (D-gun™) is similar to the HOVF devices, the difference being that the explosion of fuel and oxygen creates a detonation-pressure wave instead of the continuous steady state characteristic of the normal HVOF process [26]. However, the HVOF, HVAF and D-gun processes are characterized by high particle speeds and more energetic impacts on the substrate, leading to rather dense coatings. Especially, the IFKB lab—University of Stuttgart has been firstly develop the HVOF process with liquid feedstock, named as high-velocity suspension flame spraying (HVSFS) [30]. Moreover, comparing the coatings via HVOF technique, the HVSFS-deposited coatings are favorable for catalytic performances, such as the photodegradation application, due to more anatase phase and higher porosity [31]. The schematic diagram of HVOF and HVSFS process in Fig. 2 [29, 31].
2.2 Conventional plasma spraying
Depending on the type of plasma, these devices involve both direct-current (DC) and radio-frequency (RF) inductively coupled plasma [26]. In DC-plasma spraying, an electric arc generates a plasma within a dedicated torch. The torch mainly comprised a tungsten cathode (usually a rod or of button-type design) and a cylindrical copper anode as the nozzle, which are cooled by water during the spray. The plasma gas is injected at the base of the cathode, heated by the arc, and exits the nozzle in the form of a high-temperature, high-velocity jet. The main gases used for DC plasma spray are Ar–H2, Ar–He–H2, N2–H2 mixtures, resulting in a core temperature inside the plasma plume as high as 13,000K at the nozzle exit [21]. Figure 3 shows a schematic diagram of the DC-plasma spraying process. When the deposition is performed at atmospheric pressure, the process is commonly called atmospheric plasma spraying (APS). Currently, the torch power for the conventional APS system could be up to 50 kW, indicating possibly to melt and deposit all the materials in the earth. When it is under soft vacuum conditions, it is commonly referred to as vacuum plasma spraying (VPS), which helps avoiding external contaminations and limiting the formation of unwanted secondary phases in the coatings. Since the vacuum system is expensive, the gas shroud system has been also introduced in to the APS process to restrict the amount of entrained air and oxygen [26]. Generally, APS is used for depositing ceramic-based coatings due to the high temperature of the plasma plume, and the feedstock are normally in the form of powders of tens of micrometers size particles. For the conventional APS-deposited coatings, their characteristics mainly depending on the melting degree of particles. The states of particles inside of plasma jet could be controlled by the gas flow rate of primary gas and secondary gas, current of plasma torch, powder-feeding rate, as well as the spraying distance [32]. More recently, solution and suspension precursors as feedstock have been introduced to deposit finely structured films. The solution precursor plasma spray (SPPS) will be explored in more detail in the next section, since it is proves to be one of the most appropriate processes to prepare porous, nanostructured films.
Schematic diagram of DC plasma spraying process [29]
Another type of plasma, the RF inductively coupled plasma, is generated through the electromagnetic coupling of energy into a discharge cavity, is [21]. Like in the DC-plasma spray process, the atmosphere can be adjusted according to the targeted properties of the coatings. Compared to DC-plasma spraying, its greatest advantage is that it is well fitted for melting large particles of materials of low thermal conductivity [21]. However, this feature is not appropriate for the deposition of photocatalytic and electrocatalytic coatings, since the latter require high surface areas to maximize catalytic performances.
2.3 Liquid feedstock plasma spray (LFTS)
Nanoscale materials possess unique properties in view of photocatalytic and electrocatalytic applications [33]. In conventional plasma spray processes, the lamellae-structured coatings are formed by accumulated splats having diameters from a few tens to a few hundreds of micrometers [22]. Moreover, the major barrier faced when trying to deposit nanometer-size powders by normal thermal spraying is the difficulty of directly injecting very small particles into the high-temperature gas flow, since the momentum of the injected powder has to be of the same order as that imparted by the gas flow. In fact, it has been recognized that it is practically impossible to properly inject powder of sizes smaller than 5–10 µm [22].
This opened the question of how to form finely structured coatings, including nanostructured ones. An answer to that question was initiated in the mid-1990s with the introduction of the solution-precursor and suspension-based thermal sprays. Indeed, there are two possible routes for circumventing the drawback of the small particle size:
-
(1)
The solution precursor thermal spraying (SPTS), in which an aqueous or non-aqueous solution containing the cation(s) necessary to synthesize the desired oxide is fed into the high-temperature flame to form the final coating [21,22,23].
-
(2)
The suspension thermal spraying, in which a suspension of nanometer-sized powders is injected with a liquid rather than with a carrier gas, the nanometer-sized powders being suspended inside the liquid phase (e.g. water or ethanol) by means of dispersants [21, 22].
As far as the solution precursor thermal spray (SPTS) process is concerned, the first attempts were conducted at the State University of New York in 1997 and 1998 [33,34,35], using common flames or DC-plasma torches. Since increasing attention was paid to thermal barrier coating (TBC) and solid-oxide fuel-cell (SOFC) applications [36, 37], the use of DC plasma torches have been improved to deposit suitable microstructures starting from solution precursors, giving rise to the “solution precursor plasma spray” (SPPS) technique. As reported by M. Gell and as illustrated in Fig. 4, once the solution precursor is injected into the plasma plume, each droplet undergoes a series of chemical and physical reactions within a single step, including the evaporation of the precursor solvent, droplet breakup, precursor-solute precipitation, pyrolysis, sintering, melting, and crystallization [38]. The results clearly showed that this new SPPS technique offers a fast and facile way to deposit finely structured metal oxide films from simple solution precursors. As shown in Fig. 4, the core temperature of the plasma plume can be as high as 13,000K, a suitable level for the instantaneous decomposition and pyrolysis of any metal salt into the corresponding metal oxide. In particular, for some complex compositions, containing e.g. two distinct metallic cations, there is a high probability for a direct synthesis and deposition of binary oxides. For example, perovskite La1-xSrxMnO3 (LSM) [39] and ZnFe2O4 spinel [40] coatings were easily obtained by the SPPS method. Furthermore, the nanocrystalline structuration of the SPPS-deposited coatings can be attributed to (1) the very short flight time inside the ultrahigh-temperature plasma plume (in the order of milliseconds), which efficiently restricts the grain growth of the in-flight particles and (2) the extremely high cooling rate (106 °C/min) during the deposition of the hot particles on the substrate [41].
Chemical and physical evolution of a droplet injected into a plasma plume in SPPS process [38]
As far as the suspension thermal spray is concerned, the DC plasma spray was the earliest route explored (1996) at the University of Sherbrook [42]; the technique was then termed the “suspension plasma spray” (SPS) route. Recently, the use of HOVF sprayed suspensions has been studied to produce wear-resistant and superhydrophobic coatings [43, 44]. Like in the SPPS route, the suspensions and the surrounding droplets experience multiple physical evolutions within the high-temperature of the hot plume created either by the plasma or the combustion, but without chemical reactions [22]. However, a disadvantage of suspension based thermal spray methods is that commercial or previously synthesized nanopowders are required, meaning extra costs to procure the raw nanomaterials, lower versatility in the selection of coating compositions, and possible health issues caused by the fine particles. Indeed, the major interest of the three novel technologies lies in the flexibility in adjusting the compositions and nanostructures of the aimed metal oxides. The accumulated experience has shown that the suspension-based route (using plasma or HOVF as spraying techniques) are less suitable for functional applications than the solution precursor thermal spray (SPTS) route. Due to the horizontal deposition route, the liquid feedstock thermal sprayed coatings normally exhibited less impact of particles onto the substrates, indicating higher porosity and lower mechanical properties. Besides, since the consumption of energy for evaporation of solvent from solution or suspension, the liquid feedstock thermal sprayed coatings also exhibited lower deposition efficiencies than the techniques with solid powder and wire feedstock [23].
3 Interest of coatings deposited by thermal spray within photocatalytic and electrocatalytic applications
As stressed previously, thermal sprays techniques offer the key advantage of allowing a one-step formation of films within several minutes, in practical applications. Especially, solution precursor thermal spraying is a technique of choice to obtain tunable compositions and nanostructures, including more or less complex phases, starting simply from adequate aqueous solutions of metallic cations.
This paper section will set out the developments of thermal-sprayed photocatalytic and electrocatalytic coatings and evaluate their performances in these applications.
3.1 Photocatalytic degradation of pollutants: The contribution of thermal spray coatings
As early as 1976, the interest of the photocatalytic degradation of pollutants by TiO2 was highlighted by John H. Carey [45] who found that various organic pollutants could be degraded and mineralized into small basic molecules, essentially H2O and CO2. This form of photocatalytic treatment, based on metal-oxide semiconductors, represents an ideal method to destroy aqueous pollutants by simply exploiting the inexhaustible solar energy without consuming any chemical reagents.
Taking ZnO as an example of photocatalyst active under suitable light irradiation, the relevant processes can be summarized as follows. At first, photogenerated electron–hole pairs are formed in the valance band. If the photon energy (hν) of the irradiation light is larger than the bandgap (Eg) of ZnO, the electrons of the latter are excited and get transferred from the valence band (VB) to the conduction band (CB), leaving a positive hole in the valence band. This first process is depicted by reaction 1-i and is denoted by a yellow dashed circle in Fig. 5 [46]. The electrons become then available to react with the pollutants that are contained in the water surrounding the photocatalyst. In competition with the charge transfers that enable these chemical reactions is the recombination of electrons and holes. This recombination effect is expressed by the reaction 1-ii and can occur within the volume of the MOS catalysts (as illustrated by the path A in Fig. 5) or at their surface (path B in Fig. 5), simultaneously releasing energy in the form of heat [47, 48].
Only the remaining available electrons (e−) and holes (h+) that succeed their separation up to the external surface of the ZnO particles (as illustrated by paths C and D in Fig. 5) will participate in the photocatalytic reactions. The holes in the valence band will thus react with surface-absorbed water molecules or absorbed hydroxyl groups (OH−) to form hydroxyl radicals (•OH) as illustrated in reactions 1-iii and 1-iv, respectively [48, 49].
On the other hand, conduction-band electrons will reduce surface-absorbed oxygen atoms to form primarily superoxide radical anions (O2•−). In addition, during further steps of the photocatalytic process, other oxidizing radicals are formed, such as hydroperoxyl radical (HOO•), hydrogen peroxide (H2O2) and hydroxyl radicals (•OH) [47,48,49,50]. The corresponding processes (reactions 1-v, 1-vi, 1-vii and 1-viii) are summarized by the following reactions.
where R represents the organic compound.
Finally, due to its electrophilic nature (electron preferring), the hydroperoxyl radicals, such as •OH, can non-selectively oxidize almost all electron rich organic molecules, eventually converting them to CO2 and water (as shown in reaction 1-ix) [47]. The organic pollutant is also degraded by photogenerated holes as some of its molecules are adsorbed on the MOS particles [49]. Finally, it must be mentioned that it is still not clear which of the aforementioned reaction pathways plays the most significant role, under given experimental conditions [51].
A search performed on the “Web of Science” indicates that a total of 31 articles have been issued so far on the photodegradation of organic dyes used as surrogates of pollutants using different thermal spray processes. Their photodegradation efficiency and the corresponding test conditions are shown in Table 1, except for the papers [52] to [53]. In the reference [54], the used TiO2 membrane proved virtually inactive since the result of the blank experiment was found very close to that of TiO2. In addition, the testing device was unconventional, which raises a doubt whether some experimental artefacts produced the observed degradation effects. In [31, 55, 56], the necessary information about the photocatalytic degradation could not be found or calculated. For the photodegradation tests, the as-sprayed coatings with substrates were directly placed into a breaker or Petri dish containing organic pollutants. As such, it is feasible to do the cycling tests, without requirement of the post-filtration. Before irradiation, the liquid with samples was magnetically stirred for several hours in the dark to reach the adsorption–desorption equilibrium. During the subsequent light irradiation under room temperature, 1 mL of the liquid was extracted at regular time intervals to evaluate the remaining concentration of organic pollutants by measuring its UV–visible spectrum [57].
As shown in Table 1, TiO2 is the most studied material regardless of the spraying method used. Different photocatalytic degradation efficiencies might be related to different microstructures and film compositions as well as to different testing conditions. Except the single metal oxides (e.g. TiO2 and ZnO), the doped metal oxides (e.g. Co3+ doped ZnO), heterostructured metal oxides (e.g. CuO/ZnO), as well as binary metal oxides (ZnFe2O4) are interesting candidates as well, due to the narrowed bandgap and better separation of photogenerated holes and electrons [6, 52, 82]. For example, it can be seen that higher photodegradation activity was observed with Cu2+-TiO2 photocatalysts in [64] compared to other studies. It should be noted that the researchers ground the deposited films into a fine powder before the photocatalytic tests [64], which could be the reason for the improved performance owing to better interaction between the dye and the increased coating surface. This point was supported by [53, 70], where the finely powdered photocatalysts exhibited indeed better performance than photocatalysts produced with the same materials but in the form of coatings. However, another possible explanation for the improved performance obtained in [64] might be the formation of oxygen vacancies in the Cu-doped TiO2 films, which represents another possible means for enhancing the performances of e.g. SPPS sprayed coatings. The potential formation mechanism of oxygen vacancies from SPPS-sprayed coatings could be attributed to the typical non-equilibrium procedure and usage of reducing hydrogen gas during deposition. However, it should be mentioned that the post-heating treatment possibly to reduce the concentration of oxygen vacancies in the as-sprayed coatings [83].
Taking again TiO2 film as an example, Fig. 6 shows the morphologies produced via different spraying methods. Although different microstructures and different porosities can be expected from using different deposition routes, all the coatings were still composed of conventional flattened splats and irregular particles, meaning low surface area and consequently limited activity. Furthermore, in the APS-deposited ZnO coatings [79], the higher intensity of the X Ray diffraction signal was observed for the (101) crystallographic planes and the lower intensity for the (002) planes. This can be related to a general morphology which is not well nanostructured. Indeed, as shown in Fig. 6, all these samples seemed to be devoid of: 1) particle nanostructures (e.g. nanorods, nanowires, and nanoflakes); 2) hierarchical morphologies; and 3) preferential exposure of most active crystal planes (002). It was then suspected that SPPS route could provide better results owing to its capability of depositing hierarchical metal-oxide films with well-shaped nanostructures along with the preferential exposure of the most active crystallographic planes to the solution.
In addition, the relationships that exist between the porosity, the surface roughness and the film thickness on the one hand and the photodegradation efficiency on another hand have been investigated [17, 74]. Higher porosity and larger surface roughness induce larger contact area between the MOS and the solution, resulting in better photocatalytic performance. However, as illustrated in [17], there is actually a loose correlation between the photocatalytic degradation of organic pollutants on the one hand and the porosity as well as the coating thickness on another hand. In addition, during the APS-deposition experiments TiO2 that set out in [74], the most porous TiO2 coating was obtained using the lowest spraying power (16 kW), while the best performance was obtained for the depositions performed at the 20 kW (medium) spraying power. Finally, the porosities (i.e. the volume porosities) were calculated based on cross-section images of the coatings and included therefore the inner pores that are inaccessible and thus inoperative within photocatalytic processes. One can then infer that the coating thickness has in fact virtually no influence on the final performance. In other words, injecting lower quantities of precursors to form less thick films with better performance appeared as the preferable strategy. Another important aspect is that the performance of the photocatalytic can be limited by the wettability of the active films. This suggests that hydrophobic surfaces instead of hydrophilic ones are preferable [62].
Furthermore, for the most studied TiO2 coatings, they could be composed by anatase and rutile phases, where the anatase phase is metastable. For the TiO2 coatings deposited by various thermal spraying techniques, the P25/20 powders were employed with the anatase ratio from 75 vol.% to 80 vol.% [31, 84,85,86]. Especially, for the solution precursor thermal spraying techniques, the organic salts were employed as liquid feedstock, named as titanium isopropoxide (Ti(OCH(CH3)2)4). It was found that photocatalytic performances of TiO2 coatings are sensitive to the phase composition, where higher anatase phase improve the photocatalytic activities. The phase composition of TiO2 coatings was generally sensitive to the melting degree of the particles during spraying, where the rutile phase correspond to the fully melted particles, and the anatase structure was mostly present in the partially and non-melted particles. It was found that anatase ratio is mainly affected by the different thermal spraying sources and feedstock, where lower heating source and liquid feedstock (e.g. suspension and precursor solution) are more favorable for anatase composition [31, 84, 85]. Besides, the spraying distances, torch power, solution concentration determines the phase composition of TiO2 coatings as well [31, 86, 87]. All in all, the liquid feedstock thermal spraying techniques, including SPPS, SPS and HVSFS, could be more favorable for the photocatalytic TiO2 coatings, thanks to the more porous surface morphologies, finer structures and higher ratio of anatase phase.
ZnO is another promising photocatalyst for photodegradation, exhibiting similar bandgap structure to TiO2. Comparing with TiO2, ZnO also benefits from the following advantages: 1) ZnO is the ability to absorb a wide range of solar spectrum and more light quanta than some semiconducting [48], 2) Its various precursors were commonly inorganic salts, which are highly soluble in many solvents and highly versatile with respect to the formation of different hierarchical structures [88]. By contrast, the precursors for TiO2 were mostly organic metal salts, which are not only more expensive but more difficult to prepare due to the need for hydrolysis [48, 89]. Therefore, we decided to orient our efforts towards the development of novel hierarchical nanostructures in ZnO related coatings by using the solution precursor plasma spay (SPPS) technique as deposition technique. As shown in Fig. 7, the SPPS-deposited ZnO coatings exhibited hierarchical microstructures consisting in sea-urchin and aloe-vera like nanoparticles, developing high surface areas and efficiently exposing high activity crystal planes [90]. In the work program that was then conducted, we investigated the effect of the solute type and water pH value on the surface morphologies of SPPS-deposited ZnO coatings and we observed a high potential of that approach to form novel microstructures [90]. The brand-new hierarchical nanostructures (including nanorods and nanowires) did enhance the efficiency of photodegradation that reached 100% after120 min irradiation [57]. In addition, owing to the far-from-equilibrium conditions that prevail in plasma sprays and the further usage of hydrogen to create oxygen vacancies, we obtained highly oxygen-defective ZnO coatings which exhibited photodegradation performance even under visible light (46.2% after 360 min irradiation). This can be attributed to the narrowing of the MOS bandgap caused by the creation of oxygen vacancies [57]. Besides, in order to improve the photocatalytic activities under visible light, the heterostructured and binary metal oxides photocatalysts were also studied by SPPS techniques, where the metallic nitrates were used as the precursor solute due to their better solubility. For the heterostructured photocatalysts, we studied the CuO-ZnO, MnO-ZnO, CeO2-ZnO, Fe2O3-ZnO and Co3O4-ZnO heterostructures based on the SPPS process [58,59,60]. In these works, we endeavored to optimize the phase composition ratios in the synthesized photocatalytic coatings and we studied the influence of laminated architectures and nanorods morphologies on the photocatalytic performances [58,59,60]. For example, the use of the CeO2-ZnO system enabled to substantially increase the photodegradation efficiency which reached 62% after 360 min of irradiation under visible light [59]. It is worth stressing that it is the first time – to the authors’ knowledge—that hierarchical nano-heterostructured films including ZnO such as those shown in Fig. 7 were obtained via the SPPS route [60]. For further improving the photodegradation performances under visible light, coatings of ZnFe2O4 spinel have also been deposited and tested. It was found that such ZnFe2O4 films display improved the activity under visible light, with degradation performances of the dye climbing up to 95% within 360 min, which is more than 2 times the level of the performances of pure ZnO samples [61].
In summary, the SPPS deposition route enables the production of hierarchical nanostructures and complex phase compositions with tunable composition ratios, leading to enhanced photodegradation performances both under UV and visible light conditions.
3.2 Supercapacitor electrodes: The contribution of thermal spray coatings
Supercapacitors, also known as electrochemical electric-energy storage devices, are mainly composed of two electrode materials, two current collectors, one separator (ion conductor) and an electrolyte, as shown in Fig. 8 [91]. The essential difference between a supercapacitor and a conventional parallel-plate capacitor is the presence in the former of a solid or liquid electrolyte between the two electrodes, which is the source of ions during its energy storage process. Since the electrode material plays a decisive role in the energy storage capacity of a supercapacitor, many studies have focused on the preparation and improvement of such materials.
The schematic diagram of supercapacitors. [91]
Depending on the energy storage mechanism, supercapacitors can be classified as electric double-layer capacitors (EDLCs) or pseudocapacitors. In EDLCs, the charge is stored by way of the surface adsorption of ions from the electrolyte as a result of electrostatic attraction, which leads to the formation of two charged layers (the so-called double layer). The ELDC charge-storage mechanism is depicted in Fig. 9a. When the EDLC is charged, owing to the external circuit, cations contained in the electrolyte concentrate on the negative electrode whilst anions concentrate on the positive one, forming an EDL that compensates for the external charge imbalance. In the process of discharging, electrons travel from the negative to the positive electrode via an external circuit and both types of ions in the pores intermingle again until the cell is discharged [7]. The maximum capacitance of an EDLSC is controlled by the surface area of its active electrode and its pore-size distribution to typical values, reaching 0.15–0.4 F/m2 or ∼150 F/g1 for carbon [92]. Conway first introduced the “pseudocapacitor” in the 1970s, using electrosorption, reduction–oxidation (redox) reactions, and intercalation processes [93, 94]. Taking the RuO2 electrode as an example, its corresponding mechanism of charge storage based on reversible redox reactions within a pseudocapacitor device is illustrated by Fig. 9b. When charging, the H+ ions present in the electrolyte diffuse towards the electrode/liquid interface, driven by the external electric field, leading to reversible electrochemical reactions with the active materials (such as RuO2) on or near the surface. The H+ can further penetrate into the bulk of RuO2 during the above-mentioned redox reactions, resulting in the storage of a large amount of charges within the active materials. During the discharge, this stored charge is released as a current through the external circuit and the H+ ions return to the electrolyte solution [95]. Table 2 lists several types of charge storage reactions that occur on metal oxide electrodes of pseudocapacitors [92]. It should be noted that pseudocapacitors must feature both a double layer capacitance and a fastly reversible, superficial redox reaction, which determine the energy density while maintaining fast charge and discharge times comparable to those of EDLCs [96, 97]. Moreover, owing to the surface and bulk properties of the active materials that are used in the electrodes involved in these redox reactions, the specific capacitances of pseudocapacitors can be 10–100 times those of EDLCs. In view of the above advantages, more attention and research is being paid to pseudocapacitors.
Schematic diagram of storage mechanisms of charges in (a) EDLCs and (b) pseudocapacitance. [98]
Supercapacitor seems a fairly new topic in the sphere of thermal spray applications, since only seven publications were found. Among these articles, five were based on the SPPS route [83, 99,100,101,102] and the remaining dealt with carbon-containing electrodes that were prepared using a conventional plasma-spray methods [103, 104]. Again, it is worth reminding that the thermal spray routes benefit from rapid, one-step synthesis of binder-free films, especially when one uses the SPPS process.
Regarding the SPPS-deposited electrodes for supercapacitor applications, the University of Toronto first studied the use of MoO3 coatings as electrode material, where the ammonium molybdate tetrahydrate was used as the precursor [100]. It was found that the specific capacitance of MoO3 coatings decreased from 6.5 to 1.5 mF/cm2 when the stand-off spraying distance increased from 55 to 155 mm, which was ascribed to different morphologies of the resulting films. Based on this study, M. Golozar [101] further developed molybdenum nitride (Mo2N) coatings by reacting in a tubular furnace some MoO3 with a H2:N2 (5:1 ratio) gas mixture and pure ammonia (NH3). The electrochemical tests demonstrated a capacitance of 4.1 mF/cm2 for MoO3, while a much larger capacitance of 31 mF/cm2 was found for Mo2N under the sweep rate of 100 mV/s. During that period, nanostructured Co3O4 coatings were also developed via the SPPS process from the cobalt acetate tetrahydrate aqueous solution by University of Michigan [99]. The flexible Co3O4 electrode was deposited on a current collector made by a stainless-steel sheet (SS304). Figure 10 and Fig. 10b displaying the microstructures of these Co3O4 coatings that featured a specific capacitance of ∼162 F g−1 with a retention capacity of 72.2% after 1,000 cycles under a specific current rate of 2.75 A g−1 in 6 M KOH electrolyte. The plasma-sprayed carbon coatings exhibited a specific capacity of only 15 F/g that was obtained when the specific surface area of carbon powders was 577 m2/g [103]. This limited capacitance was likely due to the inherent limitations of carbon materials compared to metal oxides, as mentioned in Sect. 1.3.2. This point was further confirmed by a further work on a NiO/Carbon electrode, which reached 53.6 F/g as its highest specific capacitance [104].
a Photograph of the current collector of an SPPS-deposited, flexible Co3O4 electrode on a stainless steel sheet (SS304); b SEM of a Co3O4 electrode films (inset—zoom in the view). [99]
The authors also did some research work on supercapacitor electrode coatings deposited via the solution precursor thermal spraying (SPTS) techniques. Hollow micro-/nanostructures and oxygen vacancies are highly desirable for supercapacitors as they both increase the active surface area and enhance electrochemical properties, respectively. In order to benefit from both effects, pioneering investigations were conducted on SPTS-deposited NiCo2O4 coatings featuring hollow microspheres and high density of oxygen defects were. The NiCo2O4 coatings were deposited from the nickel nitrate and cobalt nitrate precursor solution. As reported in previous papers [105], surface precipitation promotes the formation of hollow particles or shell structures. This is confirmed by the Fig. 11a that illustrates the result of one of our works [71]. The electrochemical testing clearly shows that the specific capacitances enhanced and multiplied by about 20 when using oxygen-deficient NiCo2O4 electrodes containing hollow microspheres rather than NiCo2O4 electrodes composed by solid particles devoid of oxygen vacancies. Figure 11a shows indeed that the NiCo2O4 coatings composed of hollow spheres and featuring oxygen-vacancies develop a specific capacitance as high as 902 F/g at the current density of 1 A/g with a good retention of 89.2% after 2500 cycles under 20 mV/s scan rate. Based on this work, we elaborated Co3O4 coatings with different densities of oxygen vacancies and particles shapes and tested them as supercapacitor electrode materials [83]. Both cobalt nitrate and cobalt acetate precursor solution were sprayed for these Co3O4 coatings. We found that the films composed of submicron porous spheres and containing the highest content of oxygen vacancies exhibited specific capacitances as high as 1700 F/g under the scan rate of 5 mV/s. By contrast, after a heating post treatment, this value dropped down to 994 F/g due to the loss of the oxygen vacancies. Meanwhile, 96.9% of the initial capacitance was maintained after 13,000 consecutive cycles at a scan rate of 20 mV/s, as shown in Fig. 11b.
Surface morphologies and electrochemical performances of SPTS-deposited (a) NiC2O4 and (b) Co3O4 supercapacitor electrodes. [102]
In summary, starting from a review of the literature, some original works that we carried out in our laboratory have confirmed the high potential of SPTS as a technique to prepare supercapacitor electrodes exhibiting very high capacitances. These high performances are rendered possible by the favorable surface morphologies and nanostructures as well as high densities of oxygen vacancies that are created by the plasma spray techniques, especially the STPS route.
Overall, although the liquid feedstock thermal spraying techniques exhibited high potential for depositing photocatalytic and electrocatalytic coatings with fine structures, they still met several limitations. Firstly, the as-sprayed material systems are still limited to the simple metal oxides, where the composites, other metal compounds (e.g. metal sulfides, metal nitrides) and high-entropy or medium-entropy materials were lack of studies. Secondly, the novel hierarchical microstructures with well-shaped nanostructures (e.g. nanorods, nanowires) were still waiting for expanding from ZnO related coatings to other materials. Besides, the deep understanding of the influence of coating characteristics on the coating performances should be strengthen, which could supply guidance for further optimization of catalytic coatings via thermal spraying techniques. In addition, the liquid feedstock thermal sprayed coatings generally exhibited lower mechanical properties than conventional thermal spraying methods, where the higher adhesion and cohesion of coatings will be required for the industrial promotion with higher stability.
4 Summary and prospects
This paper has provided a review of the uses of thermal spray techniques for the preparation of photocatalytically and electrocatalytically active coatings involving metal oxide semiconductors and intended for photodegradation of aqueous organic pollutants and the design of supercapacitors. It also integrates original results generated in this laboratory using more specifically the SPPS technique. Regarding the photodegradation of aqueous pollutants, the most studied materials are TiO2 and ZnO based coatings, for which the existing literature reports rather weak photocatalytic activities, especially under visible light, due to the wide bandgaps of these oxides when they are involved as defect-free, large crystallites. Indeed, photocatalytic coatings deposited by usual thermal spray methods are devoid of the favorable structural properties that are of 1) well defined nanostructures, e.g. nanorods or nanowires; 2) hierarchical morphologies and 3) preferential exposure of the most active crystal planes to the aqueous medium. On the contrary, the SPPS route enables the creation of brand-new hierarchical nanostructures and composite oxide phases with tunable compositions, which greatly improves the photodegradation performances. However, further research efforts are necessary to make thermal sprayed coating a popular deposition technique of photocatalysts for the degradation of pollutants and to potentially reach the same performance levels as powdery photocatalysts, which are not environmentally, and health compatible. In the authors’ opinion, some challenges were left 1) a systematic study of the hierarchical morphologies of MOS materials as this knowledge is currently limited to ZnO based coatings, 2) the coupling of different metal oxide superconductors likely to reduce the electron/hole recombination while exhibiting at the same time well defined nanostructures.
As far as supercapacitor electrodes are concerned, the literature reveals few research works involving thermal spray deposition methods, namely because the demand for supercapacitors is relatively recent. However, most of the works identified employ solution precursor thermal spraying (SPTS) techniques. This is probably due to the strong need, in this field, of fine structures and complex phase compositions to obtain good storage performances. Here again, The SPTS route proves highly interesting for preparing supercapacitor electrodes in the form of binder-free MOS films, as it is capable of not only generating suitable surface morphologies but also creating numerous oxygen vacancies.
At the light of the present review, these challenges were still waiting for investigation for the thermal sprayed supercapacitor electrode coatings: 1) one might investigate other advanced materials rather than simple metal oxides, limited by the low electric conductivities, 2) one should gain a better understanding of the relation between electrocatalytic coatings and supercapacitor performances to orient the research towards the most suitable structures.
In summary, thermal spraying techniques own promising assets for the elaboration of highly performing photocatalytic and electrocatalytic coatings. This is especially the case for the recently developed solution precursor thermal spray route. These assets are not only in terms of the favorable compositions and nanostructures they create but also in terms of industrialization prospects, thanks to the binder-free nature and the fast and single-step fabrication process.
References
Hachem C, Bocquillon F, Zahraa O, Bouchy M (2001) Decolourization of textile industry wastewater by the photocatalytic degradation process. Dyes Pigm 49:117–125
Ollis DF, Al-Ekabi H (1993) Elsevier Science Ltd
Cai Z, Sun Y, Liu W, Pan F, Sun P, Fu J (2017) An overview of nanomaterials applied for removing dyes from wastewater. Environ Sci Pollut Res 24:15882–15904
Zhao J, Burke AF (2021) Review on supercapacitors: Technologies and performance evaluation Journal of Energy Chemistry 59:276–291
Miller JR, Simon P (2008) Electrochemical capacitors for energy management. Science Magazine 321:651–652
Casbeer E, Sharma VK, Li X-Z (2012) Synthesis and photocatalytic activity of ferrites under visible light: a review. Sep Purif Technol 87:1–14
González A, Goikolea E, Barrena JA, Mysyk R (2016) Review on supercapacitors: Technologies and materials. Renew Sustain Energy Rev 58:1189–1206
Dubal DP, Gomez-Romero P, Sankapal BR, Holze R (2015) Nickel cobaltite as an emerging material for supercapacitors: An overview. Nano Energy 11:377–399
Sk MM, Yue CY, Ghosh K, Jena RK (2016) Review on advances in porous nanostructured nickel oxides and their composite electrodes for high-performance supercapacitors. J Power Sources 308:121–140
Nithya V, Arul NS (2016) Review on α-Fe2O3 based negative electrode for high performance supercapacitors. J Power Sources 327:297–318
Cui L, Li J, Zhang X-G (2009) Preparation and properties of Co3O4 nanorods as supercapacitor material. J Appl Electrochem 39:1871
Xing W, Li F, Yan Z-F, Lu G (2004) Synthesis and electrochemical properties of mesoporous nickel oxide. J Power Sources 134:324–330
Tang W, Liu L, Tian S, Li L, Yue Y, Wu Y, Zhu K (2011) Aqueous supercapacitors of high energy density based on MoO 3 nanoplates as anode material. Chem Commun 47:10058–10060
Hsu C-T, Hu C-C (2013) Synthesis and characterization of mesoporous spinel NiCo2O4 using surfactant-assembled dispersion for asymmetric supercapacitors. J Power Sources 242:662–671
Pu J, Wang J, Jin X, Cui F, Sheng E, Wang Z (2013) Porous hexagonal NiCo2O4 nanoplates as electrode materials for supercapacitors. Electrochim Acta 106:226–234
Dey NK, Kim MJ, Kim K-D, Seo HO, Kim D, Kim YD, Lim DC, Lee KH (2011) Adsorption and photocatalytic degradation of methylene blue over TiO2 films on carbon fiber prepared by atomic layer deposition. J Mol Catal A: Chem 337:33–38
Dosta S, Robotti M, Garcia-Segura S, Brillas E, Cano IG, Guilemany JM (2016) Influence of atmospheric plasma spraying on the solar photoelectro-catalytic properties of TiO2 coatings. Appl Catal B 189:151–159
Wang H-J, Sun Y-Y, Wang C-F, Cao Y (2012) Controlled synthesis, cytotoxicity and photocatalytic comparison of ZnO films photocatalysts supported on aluminum matrix. Chem Eng J 198:154–162
Ali AM, Emanuelsson EA, Patterson DA (2010) Photocatalysis with nanostructured zinc oxide thin films: The relationship between morphology and photocatalytic activity under oxygen limited and oxygen rich conditions and evidence for a Mars Van Krevelen mechanism. Appl Catal B 97:168–181
Ni Y-H, Wei X-W, Ma X, Hong J-M (2005) CTAB assisted one-pot hydrothermal synthesis of columnar hexagonal-shaped ZnO crystals. J Cryst Growth 283:48–56
Fauchais PL, Heberlein JVR, Boulos MI (2014) Thermal spray fundamentals: from powder to part. Springer Science & Business Media
Fauchais P, Vardelle A (2012) Advanced Plasma Spray Applications, InTech
Jordan EH, Jiang C, Gell M (2015) The solution precursor plasma spray (SPPS) process: a review with energy considerations. J Therm Spray Technol 24:1153–1165
Ang ASM, Sanpo N, Sesso ML, Kim SY, Berndt CC (2013) Thermal spray maps: material genomics of processing technologies. J Therm Spray Technol 22:1170–1183
Li C-J, Ohmori A (2002) Relationships between the microstructure and properties of thermally sprayed deposits. J Therm Spray Technol 11:365–374
Gan JA, Berndt CC (2015) Thermal spray forming of titanium and its alloys. Titanium Powder Metallurgy 425–446
Li C-J, Luo X-T, Yao S-W, Li G-R, Li C-X, Yang G-J (2022) The bonding formation during thermal spraying of ceramic coatings: a review. J Therm Spray Technol 31:780–817
Li C-J (2010) Thermal spraying of light alloys. Aluminium, Magnesium and Titanium Alloys Woodhead Publishing Series in Metals and Surface Engineering. Surface Engineering of Light Alloys 184–241
Amin S, Panchal H (2016) A review on thermal spray coating processes, transfer, 2
Killinger A, Kuhn M, Gadow R (2006) High-velocity suspension flame spraying (HVSFS), a new approach for spraying nanoparticles with hypersonic speed. Surf Coat Technol 201:1922–1929
Bolelli G, Cannillo V, Gadow R, Killinger A, Lusvarghi L, Rauch J (2009) Properties of high velocity suspension flame sprayed (HVSFS) TiO2 coatings. Surf Coat Technol 203:1722–1732
Lynam A, Romero AR, Xu F, Wellman R, Hussain T (2022) Thermal spraying of ultra-high temperature ceramics: A review on processing routes and performance. J Therm Spray Technol 31:745–779
Karthikeyan J, Berndt C, Tikkanen J, Wang J, King A, Herman H (1997) Nanomaterial powders and deposits prepared by flame spray processing of liquid precursors. Nanostruct Mater 8:61–74
Karthikeyan J, Berndt CC, Reddy S, Wang JY, King AH, Herman H (1998) Nanomaterial deposits formed by DC plasma spraying of liquid feedstocks. J Am Ceram Soc 81:121–128
Karthikeyan J, Berndt C, Tikkanen J, Wang J, King A, Herman H (1997) Preparation of nanophase materials by thermal spray processing of liquid precursors. Nanostruct Mater 9:137–140
Jordan EH, Xie L, Gell M, Padture N, Cetegen B, Ozturk A, Ma X, Roth J, Xiao T, Bryant P (2004) Superior thermal barrier coatings using solution precursor plasma spray. J Therm Spray Technol 13:57–65
Padture N, Schlichting K, Bhatia T, Ozturk A, Cetegen B, Jordan E, Gell M, Jiang S, Xiao T, Strutt P (2001) Towards durable thermal barrier coatings with novel microstructures deposited by solution-precursor plasma spray. Acta Mater 49:2251–2257
Gell M, Jordan EH, Teicholz M, Cetegen BM, Padture NP, Xie L, Chen D, Ma X, Roth J (2008) Thermal barrier coatings made by the solution precursor plasma spray process. J Therm Spray Technol 17:124–135
Wang Y, Coyle T (2011) Solution Precursor Plasma Spray of Porous La1−xSrxMnO3 Perovskite Coatings for SOFC Cathode Application. J Fuel Cell Sci Technol 8:021005
Dom R, Sivakumar G, Hebalkar NY, Joshi SV, Borse PH (2012) Deposition of nanostructured photocatalytic zinc ferrite films using solution precursor plasma spraying. Mater Res Bull 47:562–570
Singh V, Karakoti A, Kumar A, Saha A, Basu S, Seal S (2010) Precursor dependent microstructure evolution and nonstoichiometry in nanostructured cerium oxide coatings using the solution precursor plasma spray technique. J Am Ceram Soc 93:3700–3708
Ravi B, Sampath S, Gambino R, Parise J, Devi P (2006) Plasma spray synthesis from precursors: Progress, issues, and considerations. J Therm Spray Technol 15:701–707
Bai M, Kazi H, Zhang X, Liu J, Hussain T (2018) Robust hydrophobic surfaces from suspension HVOF thermal sprayed rare-earth oxide ceramics coatings. Sci Rep 8:6973
Murray J, Ang A, Pala Z, Shaw E, Hussain T (2016) Suspension high velocity oxy-fuel (SHVOF)-sprayed alumina coatings: microstructure, nanoindentation and wear. J Therm Spray Technol 25:1700–1710
Carey JH, Lawrence J, Tosine HM (1976) Photodechlorination of PCB’s in the presence of titanium dioxide in aqueous suspensions. Bull Environ Contam Toxicol 16:697–701
Herrmann J-M (1999) Heterogeneous photocatalysis: fundamentals and applications to the removal of various types of aqueous pollutants. Catal Today 53:115–129
Chong MN, Jin B, Chow CW, Saint C (2010) Recent developments in photocatalytic water treatment technology: a review. Water Res 44:2997–3027
Lee KM, Lai CW, Ngai KS, Juan JC (2016) Recent developments of zinc oxide based photocatalyst in water treatment technology: a review. Water Res 88:428–448
Uddin MT (2013) Seconda^ry Metal oxide heterostructures for efficient photocatalysts, Université Sciences et Technologies-Bordeaux I
Dionysiou DD, Khodadoust AP, Kern AM, Suidan MT, Baudin I, Laîné J-M (2000) Continuous-mode photocatalytic degradation of chlorinated phenols and pesticides in water using a bench-scale TiO2 rotating disk reactor. Appl Catal B 24:139–155
Agustina TE, Ang HM, Vareek VK (2005) A review of synergistic effect of photocatalysis and ozonation on wastewater treatment. J Photochem Photobiol, C 6:264–273
Low J, Yu J, Jaroniec M, Wageh S, Al-Ghamdi AA (2017) Heterojunction photocatalysts. Adv Mater 29:1601694
Daram P, Banjongprasert C, Thongsuwan W, Jiansirisomboon S (2016) Microstructure and photocatalytic activities of thermal sprayed titanium dioxide/carbon nanotubes composite coatings. Surf Coat Technol 306:290–294
Lin Y-F, Tung K-L, Tzeng Y-S, Chen J-H, Chang K-S (2012) Rapid atmospheric plasma spray coating preparation and photocatalytic activity of macroporous titania nanocrystalline membranes. J Membr Sci 389:83–90
Bozorgtabar M, Rahimipour M, Salehi M (2010) Novel photocatalytic TiO2 coatings produced by HVOF thermal spraying process. Mater Lett 64:1173–1175
Toma F-L, Berger L, Jacquet D, Wicky D, Villaluenga I, de Miguel Y, Lindeløv J (2009) Comparative study on the photocatalytic behaviour of titanium oxide thermal sprayed coatings from powders and suspensions. Surf Coat Technol 203:2150–2156
Yu Z, Moussa H, Ma Y, Liu M, Chouchene B, Schneider R, Moliere M, Liao H (2019) Oxygen-defective ZnO films with various nanostructures prepared via a rapid one-step process and corresponding photocatalytic degradation applications. J Colloid Interface Sci 534:637–648
Yu Z, Moussa H, Liu M, Schneider R, Wang W, Moliere M, Liao H (2019) Development of photocatalytically active heterostructured MnO/ZnO and CuO/ZnO films via solution precursor plasma spray process. Surf Coat Technol 371:107–116
Yu Z, Chouchene B, Liu M, Moussa H, Schneider R, Moliere M, Liao H, Chen Y, Sun L (2020) Influence of laminated architectures of heterostructured CeO2-ZnO and Fe2O3-ZnO films on photodegradation performances. Surf Coat Technol 403:126367
Yu Z, Moussa H, Liu M, Schneider R, Moliere M, Liao H (2019) Heterostructured metal oxides-ZnO nanorods films prepared by SPPS route for photodegradation applications. Surf Coat Technol 375:670–680
Yu Z, Moussa H, Chouchene B, Liu M, Schneider R, Wang W, Moliere M, Liao H (2018) One-step synthesis and deposition of ZnFe2O4 related composite films via SPPS route for photodegradation application. Nanotechnology 30:045707
Kindole D, Ando Y (2017) Application of atmospheric solution precursor plasma spray to photocatalytic devices for small and medium industries in developing countries. Japanese Journal of Applied Physics 56:01AB10
Solonenko O, Ando Y, Nishiyama H, Kindole D, Smirnov A, Golovin A, Uehara S, Nakajima T (2018) Synthesis of thick photocatalytic titania surface layers by solution plasma spraying and subsequent treatment by pulsed laminar plasma jet. Surf Coat Technol 333:39–51
R. Kumar, S. Govindarajan, R.K. Siri Kiran Janardhana, T.N. Rao, S.V. Joshi, S (2016). Anandan, Facile one-step route for the development of in situ cocatalyst-modified Ti3+ self-doped TiO2 for improved visible-light photocatalytic activity, ACS appl mater interfaces. 8:27642–27653
Huang J, Gong Y, Liu Y, Suo X, Li H (2017) Developing titania-hydroxyapatite-reduced graphene oxide nanocomposite coatings by liquid flame spray deposition for photocatalytic applications. J Eur Ceram Soc 37:3705–3711
Liu Y, Huang J, Ding S, Liu Y, Yuan J, Li H (2013) Deposition, characterization, and enhanced adherence of escherichia coli bacteria on flame-sprayed photocatalytic titania-hydroxyapatite coatings. J Therm Spray Technol 22:1053–1062
Toma F-L, Berger L-M, Shakhverdova I, Leupolt B, Potthoff A, Oelschlägel K, Meissner T, Gomez JAI, de Miguel Y (2014) Parameters influencing the photocatalytic activity of suspension-sprayed TiO 2 coatings. J Therm Spray Technol 23:1037–1053
Liu X, Wen K, Deng C, Yang K, Deng C, Liu M, Zhou K (2018) Nanostructured Photocatalytic TiO2 Coating Deposited by Suspension Plasma Spraying with Different Injection Positions. J Therm Spray Technol 27:245–254
Kozerski S, Toma F-L, Pawlowski L, Leupolt B, Latka L, Berger L-M (2010) Suspension plasma sprayed TiO2 coatings using different injectors and their photocatalytic properties. Surf Coat Technol 205:980–986
Yi Z, Guofeng C, Ma W, Wei W (2008) Effect of external bias voltage and coating thickness on the photocatalytic activity of thermal sprayed TiO2 coating. Prog Org Coat 61:321–325
Garcia-Segura S, Dosta S, Guilemany JM, Brillas E (2013) Solar photoelectrocatalytic degradation of Acid Orange 7 azo dye using a highly stable TiO2 photoanode synthesized by atmospheric plasma spray. Appl Catal B 132:142–150
Bordes MC, Vicent M, Moreno A, Moreno R, Borrell A, Salvador M, Sánchez E (2013) Microstructure and photocatalytic activity of APS coatings obtained from different TiO2 nanopowders. Surf Coat Technol 220:179–186
Bordes MC, Vicent M, Moreno A, López V, Moreno R, Benavente R, Sánchez E (2014) Preparation of feedstocks from nano/submicron-sized TiO2 particles to obtain photocatalytic coatings by atmospheric plasma spraying. Ceram Int 40:16213–16225
Zhang C, Chaudhary U, Das S, Godavarty A, Agarwal A (2013) Effect of Porosity on Photocatalytic Activity of Plasma-Sprayed TiO2 Coating. J Therm Spray Technol 22:1193–1200
Bordes MC, Vicent M, Moreno R, García-Montaño J, Serra A, Sánchez E (2015) Application of plasma-sprayed TiO2 coatings for industrial (tannery) wastewater treatment. Ceram Int 41:14468–14474
Colmenares-Angulo J, Zhao S, Young C, Orlov A (2009) The effects of thermal spray technique and post-deposition treatment on the photocatalytic activity of TiO2 coatings. Surf Coat Technol 204:423–427
Bozorgtabar M, Salehi M, Rahimipour M, Jafarpour M (2010) Influence of high velocity oxy-fuel parameters on properties of nanostructured TiO2 coatings. Bull Mater Sci 33:671–675
Navidpour A, Kalantari Y, Salehi M, Salimijazi H, Amirnasr M, Rismanchian M, Siahkali MA (2017) Plasma-Sprayed Photocatalytic Zinc Oxide Coatings. J Therm Spray Technol 26:717–727
Su C, Lu C, Hsiao W, Liu W, Shieu F (2013) Evaluation of the microstructural and photocatalytic properties of aluminum-doped zinc oxide coatings deposited by plasma spraying. Thin Solid Films 544:170–174
Navidpour A, Salehi M, Salimijazi H, Kalantari Y, Siahkali MA (2017) Photocatalytic Activity of Flame-Sprayed Coating of Zinc Ferrite Powder. J Therm Spray Technol 26:2030–2039
Navidpour A, Salehi M, Amirnasr M, Salimijazi H, Siahkali MA, Kalantari Y, Mohammadnezhad M (2015) Photocatalytic Iron Oxide Coatings Produced by Thermal Spraying Process. J Therm Spray Technol 24:1487–1497
Samadi M, Zirak M, Naseri A, Khorashadizade E, Moshfegh AZ (2016) Recent progress on doped ZnO nanostructures for visible-light photocatalysis. Thin Solid Films 605:2–19
Yu Z, Ma Y, Liu M, Huang X, Song C, Song G, Liao H, Chen Y, Sun L (2021) Oxygen-deficient Co3O4 submicron porous sphere films as highly active supsercapacitor electrodes. Surf Coat Technol 405:126513
Bozorgtabar M, Rahimipour M, Salehi M (2010) Effect of thermal spray processes on anatase–rutile phase transformation in nano-structured TiO2 photo-catalyst coatings. Surf Eng 26:422–427
Toma F-L, Sokolov D, Bertrand G, Klein D, Coddet C, Meunier C (2006) Comparison of the photocatalytic behavior of TiO 2 coatings elaborated by different thermal spraying processes. J Therm Spray Technol 15:576–581
Lee C, Choi H, Lee C, Kim H (2003) Photocatalytic properties of nano-structured TiO2 plasma sprayed coating. Surf Coat Technol 173:192–200
Simfroso K, Cabo SR, Unabia R, Britos A, Sokołowski P, Candidato R Jr (2023) Solution Precursor Plasma Spraying of TiO2 Coatings Using a Catalyst-Free Precursor. Materials 16:1515
Kumar SG, Rao KK (2015) Zinc oxide based photocatalysis: tailoring surface-bulk structure and related interfacial charge carrier dynamics for better environmental applications. RSC Adv 5:3306–3351
Daneshvar N, Salari D, Khataee A (2004) Photocatalytic degradation of azo dye acid red 14 in water on ZnO as an alternative catalyst to TiO2. J Photochem Photobiol, A 162:317–322
Yu Z, Moussa H, Liu M, Chouchene B, Schneider R, Wang W, Moliere M, Liao H (2018) Tunable morphologies of ZnO films via the solution precursor plasma spray process for improved photocatalytic degradation performance. Appl Surf Sci 455:970–979
Wu Z-S, Feng X, Cheng H-M (2014) Recent advances in graphene-based planar micro-supercapacitors for on-chip energy storage. Natl Sci Rev 1:277–292
Zhi M, Xiang C, Li J, Li M, Wu N (2013) Nanostructured carbon–metal oxide composite electrodes for supercapacitors: a review. Nanoscale 5:72–88
Simon P, Gogotsi Y, Dunn B (2014) Where do batteries end and supercapacitors begin? Science 343:1210–1211
Augustyn V, Simon P, Dunn B (2014) Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ Sci 7:1597–1614
Long JW, Bélanger D, Brousse T, Sugimoto W, Sassin MB, Crosnier O (2011) Asymmetric electrochemical capacitors—Stretching the limits of aqueous electrolytes. MRS Bull 36:513–522
Wang G, Zhang L, Zhang J (2012) A review of electrode materials for electrochemical supercapacitors. Chem Soc Rev 41:797–828
Jost K, Dion G, Gogotsi Y (2014) Textile energy storage in perspective. Journal of Materials Chemistry A 2:10776–10787
Zhang X, Cheng X, Zhang Q (2016) Nanostructured energy materials for electrochemical energy conversion and storage: A review. J Energy Chem 25:967–984
Tummala R, Guduru RK, Mohanty PS (2012) Nanostructured Co3O4 electrodes for supercapacitor applications from plasma spray technique. J Power Sources 209:44–51
Golozar M, Chien K, Coyle TW (2012) Orthorhombic α-MoO3 Coatings with Lath-Shaped Morphology Developed by SPPS: Applications to Super-Capacitors. J Therm Spray Technol 21:469–479
Golozar M, Chien K, Lian K, Coyle TW (2013) Pseudo-Capacitors: SPPS Deposition and Electrochemical Analysis of α-MoO3 and Mo2N Coatings. J Therm Spray Technol 22:710–722
Ma Y, Yu Z, Liu M, Song C, Huang X, Moliere M, Song G, Liao H (2019) Deposition of binder-free oxygen-vacancies NiCo2O4 based films with hollow microspheres via solution precursor thermal spray for supercapacitors. Ceram Int 45:10722–10732
Kavaliauskas Ž, Marcinauskas L, Aikas M, Valinčius V, Milieška M, Baltušnikas A (2017) Effect of carbon powder thermal activation on characteristics of supercapacitor electrodes. J Electrostat 90:61–66
Kavaliauskas Z, Marcinauskas L, Valatkevicius P (2011) Formation and characterization of carbon and nickel oxide/carbon composites for supercapacitors. Acta Phys Pol, A 119:253–255
Saha A, Seal S, Cetegen B, Jordan E, Ozturk A, Basu S (2009) Thermo-physical processes in cerium nitrate precursor droplets injected into high temperature plasma. Surf Coat Technol 203:2081–2091
Acknowledgements
The authors would like to acknowledge the project supported by the Natural Science Foundation of Jiangsu Higher Education Institution of China (Grant No. 20KJB430003), the open project of Key Laboratory of Green Fabrication and Surface Technology of Advanced Metal Materials (Grant No. GFST2021KF01), and Humboldt Fellowship for Postdoctoral Researcher (Dr. Zexin YU).
Author information
Authors and Affiliations
Contributions
All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yu, Z., Wang, B., Gui, L. et al. Development of photocatalytic and electrocatalytic coatings via thermal spraying for environmental and energy storage applications: a short review. Surf. Sci. Tech. 2, 7 (2024). https://doi.org/10.1007/s44251-023-00030-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44251-023-00030-5