Abstract
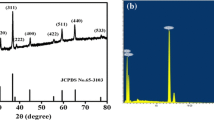
Co3O4 nanorods have been successfully synthesized by thermal decomposition of the precursor prepared via a facile and efficient microwave-assisted hydrothermal method, using cetyltrimethylammonium bromide (CTAB) with ordered chain structures as soft template for the first time. The obtained Co3O4 was characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), and electrochemical measurements. The results demonstrate that the as-synthesized nanorods are single crystalline with an average diameter of about 20 to 50 nm and length up to several micrometers. Preliminary electrochemical studies, including cyclic voltammetry (CV), galvanostatic charge–discharge, and electrochemical impedance spectroscopy (EIS) measurements, are carried out in 6 M KOH electrolyte. Specific capacitance of 456 F g−1 for a single electrode could be achieved even after 500 cycles, suggesting its potential application in electrochemical capacitors. This promising method could provide a universal green chemistry approach to synthesize other low-cost and environmentally friendly transition metal hydroxide or oxide.







Similar content being viewed by others
References
Conway BE (1999) Electrochemical supercapacitors. Kluwer Academic/Plenum Publishers, New York
Subramanian V, Zhu H, Vajtai R, Ajayan PM, Wei B (2005) J Phys Chem B 109:20207
Sarangapani S, Tilak BV, Chen CP (1996) J Electrochem Soc 143:3791
Zheng JP, Jow TR (1995) J Electrochem Soc 142:6
Kuo C, Mare AA (1996) J Electrochem Soc 143:124
Yuan CZ, Gao B, Su LH, Zhang XG (2008) Solid State Ion 178:1859
Cao L, Zhao YK, Lu M, Li HL (2003) J Chin Sci Bull 48:1212
Liu Y, Zhao WW, Zhang XG (2008) Electrochim Acta 8:3296
Wang Y, Zhang WS, Evans DG, Duan X (2005) J Electrochem Soc 152:A2130
Chang JK, Lin CT, Tsai WT (2004) Electrochem Commun 6:666
Pang SC, Anderson AM, Chapman TW (2000) J Electrochem Soc 147:444
Zhao DD, Bao SJ, Zhao WJ, Hu HL (2007) Electrochem Commun 9:869
Cao L, Xu F, Liang YY, Li HL (2004) Adv Mater 16:1853
Natile MM, Glisenti A (2003) Chem Mater 15:2502
Wang X, Chen XY, Gao LS, Zheng HG, Zhang ZD, Qian YT (2004) J Phys Chem B 108:16401
Cao AM, Hu JS, Liang HP (2006) J Phys Chem B 110:15858
Barrera E, Viveros T, Montoya A, Ruiz M (1999) J Sol Energy Mater Sol Cells 57:127
Feng J, Zeng HC (2003) Chem Mater 15:2829
Palmas S, Ferrara F, Vacca A, Mascia M, Polcaro AM (2007) Electrochim Acta 53:1439
Zhang XJ, Li QL (2008) Mater Lett 62:988
Zhu YJ, Wang WW, Qi RJ, Hu XL (2004) Angew Chem Int Ed Engl 43:1410
Yu WY, Tu WX, Liu HF (1999) Langmuir 15:6
Zhang XJ, Jiang W, Song D, Liu JX, Li FS (2008) Mater Lett 62:2343
Liu YK, Wang GH, Xu CK, Wang WZ (2002) Chem Commun 1486
Beach E, Brown S, Shqau K, Mottern M, Warchol Z, Morris P (2008) Mater Lett 62(12–13):1957
Dong LH, Chu Y, Liu Y, Li MY, Yang FY, Li LL (2006) J Colloid Interface Sci 301:503
Palmas S, Ferrara F, Vacca A, Mascia M, Polcaro AM (2007) Electrochim Acta 53:400
Acknowledgements
This study has been supported by the National Natural Science Foundation of China (No.20663006). We also thank Scientific Research Program of the Higher Education Institution of Xinjiang (XJEDU2006S206) for partial support of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cui, L., Li, J. & Zhang, XG. Preparation and properties of Co3O4 nanorods as supercapacitor material. J Appl Electrochem 39, 1871–1876 (2009). https://doi.org/10.1007/s10800-009-9891-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-009-9891-5