Abstract
Introduction
Point of care viral load (POC VL) testing improves viral suppression and retention in HIV care, and is increasingly being integrated into routine health services for African pregnant women living with HIV. We examined processes of implementing POC VL testing in antenatal care and at delivery for Ugandan mothers living with HIV as part of a pilot randomized trial (Clinical Trial Number: NCT05092997).
Methods
We conducted individual qualitative interviews with 12 clinical and research staff who implemented POC VL testing and 22 mothers who received POC VL testing using the Xpert® HIV-1 Viral Load Assay (Cepheid Inc., Sunnyvale, CA, USA). An inductive, content analytic approach was used to examine the interview transcripts. The analysis addressed the question: How did a group of Ugandan health care providers approach the process of implementing POC VL testing in antenatal care and at delivery for mothers living with HIV?
Results
The analysis yielded three themes. (1) Staff created an efficient system of communication and then relied on that system to coordinate testing procedures. (2) They also found ways of increasing the speed and efficiency of the testing process. (3) They adopted a “mother-centered” approach to implementation, prioritizing the needs, preferences, and well-being of women in planning and carrying out testing procedures.
Conclusion
As POC VL testing becomes more widely used across high HIV burden settings, understanding how implementers approach the implementation process and what they do to make an intervention successful will be an important part of evaluating feasibility.
Clinical Trial Number: NCT05092997.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Viral suppression during pregnancy and postpartum sustains the health of mothers living with HIV and reduces risk of HIV transmission to newborns [1, 2]. Viral suppression requires adherence to antiretroviral therapy (ART), yet suboptimal adherence and disengagement from HIV care are common among pregnant women, especially after giving birth [3,4,5,6].
Point of care viral load (POC VL) testing reduces turnaround time from sample collection to availability of results [7,8,9,10], and increases rates of viral suppression and retention in care [7, 11]. Studies comparing results of POC with laboratory-based viral load testing in diverse African health care settings confirm POC VL test results are accurate and reliable [10, 12, 13].
Uganda adopted viral load testing as the preferred means of monitoring response to HIV treatment in 2015 [14]. Currently, mothers living with HIV and taking ART receive viral load testing at the initial antenatal care (ANC) visit and every three months thereafter throughout pregnancy and breastfeeding. Newly diagnosed mothers receive VL testing at the initial ANC visit if CD4 test results reveal CD4 to be < 200 cells/mm3. For virally suppressed mothers, the test is then repeated every three months throughout pregnancy and until cessation of breastfeeding [15].
Presently, blood samples collected for VL testing at local clinics in Uganda are routed through a network of “hub” facilities to central health laboratories for processing, and the results returned to clinics via the same network [16]. Once test results have arrived at the clinic, they are typically reported to patients at the next follow-up visit. The entire process can take weeks or months, delaying needed clinical action.
A few studies have evaluated the impact of POC VL testing on viral suppression rates for pregnant and postpartum women in Africa, with mixed results. Delivery of POC VL testing with enhanced viral load counseling and phone follow-up by peer mothers substantially increased rates of viral suppression for pregnant and postpartum women in a recent study taking place in rural southwest Uganda [17]. Other evaluations report no statistically significant effect [9, 18].
POC viral load testing is increasingly being integrated into routine antenatal care and as part of delivery for African pregnant women living with HIV [13, 19]. To inform this process, we offer results from an analysis of qualitative data addressing the question: How did a group of Ugandan health care providers (“implementers”) approach the process of implementing POC VL testing in antenatal care and at delivery for mothers living with HIV? The analysis was part of an intervention development study of POC VL testing and HIV treatment outcomes for pregnant and postpartum women taking place in Kampala, Uganda (The Kingasa Study).
2 Methods
2.1 Study design
The Kingasa Study (“It benefits me”, in Luganda, the local language) was a multi-method pilot randomized trial using a factorial design, in which 151 Ugandan pregnant women living with HIV who were at least 18 years of age, had partners of unknown HIV status, and were receiving antenatal care were randomized to an intervention intended to improve HIV treatment outcomes or standard of care (SOC). The intervention consisted of POC HIV-1 viral load testing with same day adherence counseling for participating women, and an invitation to visit the clinic for HIV testing and other preventive screenings for their male partners. SOC consisted of laboratory-based viral load testing for women with reporting of results at the next clinic visit, following Ugandan National Guidelines [15] (Clinicaltrials.gov: NCT05092997).
Questionnaire data were collected from women every three months until three months postpartum. Sociodemographic information was collected at baseline; information on birth outcomes and self-reported ART adherence were collected at follow-up. The primary outcome of the trial was viral suppression at three months postpartum. Trial results are reported elsewhere [20].
2.2 Setting
The Kingasa Study took place at a Level III public health clinic in Kampala, Uganda. More than 26,000 ANC visits took place at the clinic in 2022–2023 [21]. The clinic also offers general outpatient services and HIV testing and treatment. POC HIV-1 VL testing for mothers participating in Kingasa took place at enrollment, delivery, and at three months postpartum. Blood draws for testing were carried out principally by laboratory technicians, but also by nurses and midwives. Sample collection took place in consulting rooms, in or near the clinic’s maternity ward, and at offsite locations (participants’ homes, other clinical facilities). Samples were processed using the Xpert® HIV-1 Viral Load Assay (Cepheid Inc., Sunnyvale, CA, USA) located in the clinic laboratory. The Xpert Assay processes up to four blood samples simultaneously, producing results approximately 90 min after samples are loaded. Laboratory technicians processed the samples, and handed the results off to nurses, who reported them to patients.
2.3 Qualitative data collection
The Kingasa Study included a qualitative component in which data were collected from two groups: (1) clinical and research staff who implemented the intervention (“implementers”), and (2) a convenience sample of mothers who received POC HIV-1 VL testing using the Xpert Assay. Single in-depth qualitative interviews were carried out with all participants using semi-structured interview guides by authors BK, AN, and VK. Participants were contacted by telephone by interviewers to explain the research and the meaning of participation and set up an interview appointment. Implementer interviews covered aspects of personal and professional background, knowledge and perceptions of POC VL testing, and experiences of implementing POC VL testing in Kingasa. Mothers’ interviews covered aspects of personal background, quality of the partnered relationship, and attitudes, perceptions and experiences of POC VL testing. Interviews took place in mutually agreeable, private locations in the local language (Luganda) or in English. They were audio-recorded and transcribed in English as soon as possible after each interview was completed. Interviews lasted about an hour. Qualitative interviews were carried out beginning in December 2021 and ending in September 2022.
2.4 Qualitative data analysis
An inductive, content analytic approach was used to examine the interview transcripts for the analysis reported here [22]. We reviewed all the transcripts to identify and extract content relevant to process—what interviewees said about how the Kingasa intervention was implemented. Relevant content was grouped iteratively through repeated readings into conceptual clusters based on similarities. We then grouped clusters to form larger thematic concepts. Descriptive categories were developed to represent the concepts. To develop descriptive categories, we named, specified, and illustrated the concepts using excerpts from the data. For this analysis, data were not formally coded. The analysis yielded three categories reported as Results, below.
3 Results
3.1 Characteristics of participants
3.1.1 Implementer participants
Twelve of 16 clinical and research staff who implemented the Kingasa intervention took part in interviews for the qualitative study. The remainder declined to participate. Six implementer interviewees were women; six were men. Three-quarters had university-level educations. Half (N = 6) were laboratory technicians; five were nurses and/or counselors; one was a midwife. Median age of implementer interviewees was 32 years (See Table 1).
3.1.2 Participating mothers
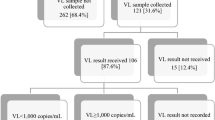
Twenty-two (15%) of mothers participating in the Kingasa Study took part in interviews for the qualitative component. Median age was 27.5 years. Half (50%, N = 11) had at least some secondary education; half (50%, N = 11) were working to earn income.
Mothers reported being with their current male partners for a median of three years (IQR: 1.75 to 6). Median total reported pregnancies was four (IQR: 3–5); median reported number of living children was three (IQR: 2–4). Median reported years since HIV diagnosis was four (IQR: 2–6.98). All mothers reported taking ART at the time of participation. Nearly two-thirds (64%, N = 14) reported having disclosed their HIV status to their current partner. Results of POC HIV-1 VL testing at enrollment revealed all but one of the mothers to be virally suppressed (95%, N = 21); at delivery all were virally suppressed (< 50 copies/ml) (See Table 2).
3.2 POC HIV-1 VL testing implementation
In qualitative interviews, half (50%, N = 11) of mothers reported receiving results of their POC HIV-1 VL testing at enrollment on the same day as sample collection. Half (50%, N = 11) of births took place at the study clinic. The remainder took place at other health facilities. Slightly fewer than half of blood draws for POC VL testing at delivery were completed at the study clinic (41%, N = 9); about a third (37%) were done at home. All blood draws described by mothers were reported to have taken place after, rather than before, the birth (See Table 3).
3.3 Qualitative results
Below we present the three thematic descriptive categories resulting from the inductive content analysis: 1. Coordination and Communication; 2. Speed and Efficiency; and 3. “Mother-centeredness”.
3.3.1 Coordination and communication
Implementers emphasized coordination and communication in their accounts of carrying out POC viral load testing. For example, they created a secure, instant messaging chat group, and used it to keep each other updated in real time.
When a mother presented at the clinic for delivery, midwives alerted colleagues via the chat group. Staff then met to assess the mother’s condition and decide on the best time and location to collect a blood sample for POC VL testing. They felt blood draws could be carried out in the maternity ward, the lab, or another nearby location offering privacy. For implementers, optimal moments for drawing blood at delivery were either early in labor or 30–60 min after the birth. The actual timing varied with the mother’s well-being, preferences, and circumstances.
About half (45%) of women in the qualitative sample delivered at a health facility outside the study clinic. This complicated sample collection for POC VL testing for implementers, as medical staff at the external sites had to give permission for a visitor to carry out a procedure involving a patient under their care. Multiple interactions and/or a letter of introduction were usually required to obtain permission. Rather than struggle with these coordination and communication challenges, implementers often chose to meet mothers in their homes for blood draws. This usually meant testing was completed a few days after delivery.
Blood draws at home also entailed extensive coordination and communication. Multiple phone calls were required to arrange appointments, get directions, and ensure all relevant parties were informed and kept up to date. The process usually began with a call from an implementer to the mother confirming permission for a home visit and fixing a time to meet. The caller explained that the blood draw would be done by a laboratory technician, whom the mother likely had not previously met. Following the conversation, the caller contacted laboratory staff to inform them a home visit was needed. The technician making the visit would then follow up with another call to the mother to introduce himself or herself, confirm agreement, and get directions. The technician would sometimes need to call again while en route for directions or to confirm the time was still convenient. One interviewee described the process this way:
“…the mother [must be] informed that someone is coming to take off samples… You cannot just come out to meet her and be like, ‘I have been told to take off samples from you.’ She must have gotten the information prior and she is expecting you. [At the same] time we must keep in touch with those people that sent us to the field. Even when I reach the mother, before taking off samples I call and tell a research staff that I have arrived…” Laboratory Technician
Home visits to mothers who had not disclosed to partners could be particularly challenging. Careful planning and coordination were required to avoid encountering partners during the visit. If this proved impossible, the visitor had to find a way of explaining why s/he was there and carry out the procedure, while at the same time avoiding unwanted disclosure. One home visitor recounted this experience:
“When I got there, they welcomed me. The husband gave me a seat and we started conversing. The man had a lot of questions. I told him, ‘when these mothers come to us at the facility we always encourage them to come with you [partner] … but you did not come. So, allow me to explain to you why we are following [your wife]’. But I did not disclose to him that she is HIV positive. … I told him that we followed her home because she did not deliver from [study clinic] … and she was discharged without us knowing what packages were given to her and how she was faring. So that is why I am here….” Laboratory Technician
Implementer coordination and communication were facilitated by access to mobile phone technology.
3.3.2 Speed and efficiency
POC VL testing was known as “the quick, 90-min test” at the study clinic. Implementers introduced the test to mothers as taking 90 min from blood draw to receipt of results. In fact, 90 min was the time required for the Xpert Assay to process a sample once it had been loaded into the system. The time needed to collect the sample, transport it to the lab, complete preliminary procedures, and hand the results off for reporting to a waiting mother lengthened total testing time. Implementers recommended lengthening estimates of the time required from blood draw to receipt of results, when introducing POC VL testing to mothers:
“I would not tell [mothers] that [POC VL testing] takes 90 minutes because it exceeds that... I think it should be like two hours. There should be an allowance for things that happen in between.” Nurse
Implementers accepted the expectation that POC VL test results would be available in 90 min as part of the Kingasa intervention. To meet this expectation, they set a brisk pace for activities associated with testing. Implementers developed a number of strategies to increase the speed and efficiency with which samples could be collected, analyzed, and the results returned to mothers. They used the instant messaging group chat to keep in touch so potential gaps between steps in the process could be minimized, and built redundancy into the distribution of skills, roles, and functions needed to complete the testing process. This saved time by enabling staff to fill in for each other to complete required tasks. One implementer described it this way:
“[What] we did was to empower our midwives and nurses. We had a session and taught them how to collect the samples properly. … It was something they took on, and at some point, they were collecting the samples and we would just do checks on them to see that they were properly collected.” Laboratory Technician
Often implementers had to stretch themselves to keep up the pace. To shorten turnaround time, for example, technicians might return to the laboratory when they were off duty, in order to process samples and produce results as quickly as possible. Technicians also worked after hours to finish processing samples in a single day. One technician described the following experience:
“I went to [off-site location] to collect the sample. I remember I left [the clinic] at 2:00 pm and by 2:30 pm I was there. I left by 6:00 pm and I had to come back and run the samples that very evening because we are supposed to run them there and then. I left [the lab] around 8:00 pm to go back home.” Laboratory Technician
Despite efforts to maximize efficiency, mothers sometimes waited several hours at the clinic to receive their POC VL testing results. Implementers took steps to make the waiting time easier by providing snacks and/or a quiet space to rest. Sometimes, mothers could not wait long enough to receive their results in person. In these cases, they returned to clinic at a later date, or arranged with staff to receive their results over the phone. One mother explained her experience:
“…study staff drew a blood sample … and that sample was taken down [to the laboratory] for testing. That same study staff came back and said that there was no electricity and I was told that I am going to wait longer. While I was waiting, it started raining and I told them that I have to go before it is heavy rain. Study staff told me that it is okay, they will contact me on phone and tell me my results. One hour after I had reached home, I was called and study staff told me that the results have showed that my blood is good…”. Mother
3.3.3 “Mother-centeredness”
Implementers’ descriptions of their experiences in carrying out POC VL testing suggest they placed mothers at the center of the intervention, letting their needs and preferences drive the implementation process wherever possible. We have seen this in how they carried out home visits for blood draws, when visiting laboratory technicians checked in repeatedly with mothers en route, and avoided approaching a residence until receiving explicit permission to do so. We see it also in the emphasis they placed on offering choices to mothers. The choice between receiving POC VL testing results in person on the same day, in person on a subsequent day, or over the phone is one example of this:
“You agree with the mother on how she wants to receive the results. … We can always give clients options that are flexible, because you are not going to make the mother sit there and wait for results when she has a baby at home and has not had lunch. … Some mothers come to the facility, so they can walk in. A mother can also give you her phone number if she has one. You ask what time she wants to be called and you record it and call her…” Nurse
Kingasa implementers’ “mother-centered” approach is also evident in the investments they made in providing support and promoting mothers’ well-being. An example is the practice of contacting mothers by phone between clinic appointments to check on their pregnancy and HIV-related needs. Mothers felt cared for as a result. Said one mother after receiving home delivery of an ART refill:
“I really got support from Kingasa Study because there was even a time I was sick and the study staff brought me medicine (ART) at home. I simply called them and they had no problem bringing me ART at home. The calls they made asking me how I am after I gave birth also showed that they were caring for me.” Mother
While adhering to the intervention protocol, implementers eschewed a “one-size-fits-all” approach to implementation, opting instead to individually assess situations and tailor intervention activities accordingly. The care taken to understand each mother’s situation when choosing a moment for blood draw at delivery testifies to this. One implementer characterized Kingasa’s individualized, “in-the-moment” approach to caring for mothers this way:
“...we should not get used to thinking people are the same. [We should] treat everyone the way they are, … the way they have come, and not ever think that someone’s experience is the same as someone else’s…Every client is unique and they all go through different things. If you think what you dealt with yesterday is what you will deal with today that is when we lose it. … Let us give them a kind of holistic environment and treat them as they come, not as they were yesterday.” Nurse
4 Discussion
Our inductive content analysis of implementers’ and mothers’ qualitative accounts yielded three themes that speak to how health care staff worked to implement the Kingasa POC HIV-1 VL testing intervention. They created an efficient system of communication and then relied on that system to coordinate POC VL testing procedures. They looked for and found ways of increasing the speed and efficiency of the testing process. And they prioritized the needs, preferences, and well-being of participating mothers in planning and carrying out testing procedures and reporting results.
This qualitative evaluation privileges the voices of implementers. Implementer voices have been represented in a few other studies focusing on POC VL testing, usually to report perceived benefits and challenges, and/or suggestions for how testing can be improved [23,24,25]. In this study, we asked implementers to describe how they carried out testing activities. As a result, we learned what implementation was like for them – how they approached it, its impact on established ways of working, and adjustments they made to ensure testing procedures were completed successfully.
To date, most studies examining the feasibility of POC VL testing in African health care settings have focused on implementation outcomes, e.g., number of tests completed daily, accuracy of test results, and/or turnaround time to receipt of results [10, 12, 13, 19]. In contrast, our study was process-oriented. Our process orientation offers a complementary perspective from which to evaluate feasibility, asking questions about how implementers organized to make POC VL testing happen. Both outcome and process-oriented evaluations are essential to an accurate and thorough understanding of feasibility.
In implementation science, adaptation has been defined as “a process of thoughtful and deliberate alteration to the design or delivery of an intervention, with the goal of improving its fit or effectiveness in a given context” [26]. A significant adaptation made to the Kingasa POC VL testing intervention in the process of implementation was the use of home visits to draw blood for testing at delivery for mothers who gave birth outside the study clinic.
The Enhanced Framework for Reporting Adaptations and Modifications (FRAME) specifies a set of parameters for the characterization of adaptations, in the interests of promoting understanding of the kinds of adaptations that take place, and how and when they occur [26]. Applying FRAME parameters, we may characterize the home visit adaptation in Kingasa as an unplanned modification to delivery of POC VL testing. The adaptation was “unplanned” in the sense that it represented a solution to an unanticipated problem that came up during the implementation process, i.e., the difficulty of collecting blood samples while mothers were inpatients at another clinical facility. Potential risks to confidentiality and the possibility of non-voluntary disclosure of HIV status as part of the home visit adaptation were recognized by implementing staff, who worked to minimize these risks in ways described here while trying to ensure the benefits of POC viral load testing were made available. The home visit adaptation was intended to facilitate continued delivery of the Kingasa intervention’s core component of POC VL testing, making it “fidelity consistent,” in FRAME terms.
Home visits for blood draws were a way of maximizing the number of Kingasa mothers who would receive and benefit from POC VL testing at delivery. At the same time, the effort appears to have been resource-intensive, requiring relatively large investments of time and money to implement. Whether home visits for sample collection enhance or detract from the feasibility of POC VL testing for pregnant women at delivery is a question that merits future study.
Several suggestions for optimizing implementation of POC VL testing for pregnant women emerged from our analysis. One suggestion from implementers was to introduce POC VL testing in a way that sets realistic expectations for turnaround time. Including time required to complete the entire testing process in descriptions to recipients will reduce pressure on implementers and frustration for individuals awaiting their results. A second suggestion was to build in overlap in implementer roles and functions when planning the implementation process. In Kingasa, for example, implementers described how training midwives alongside laboratory technicians to perform blood draws accelerated sample collection. Ensuring a reliable communication system is in place was a third suggestion for optimizing implementation of POC VL testing for pregnant women living with HIV.
This study contributes to research on intervention implementation process by detailing how one group of health care professionals made POC VL testing happen for Ugandan pregnant women living with HIV and receiving antenatal care in a Level III public health clinic. We recognize the study’s limitations. Women qualitative participants comprised a convenience sample; however, this analysis prioritized understanding the experiences of implementers. Because Kingasa was a research study, we do not claim the approach Kingasa staff took matches the approach implementers might take outside a controlled research context. Finally, we acknowledge that implementer accounts of their activities in interviews may not correspond completely to “what really happened.” Data from direct observations of POC VL testing implementation in addition to individual interviews would have allowed for triangulation of two data sources. The additional perspective on implementation process provided by two data sources would have strengthened the validity of analytic results.
5 Conclusion
As POC VL testing becomes more widely used across high HIV burden settings, understanding how implementers think about and approach the implementation process and what they do to make an intervention successful will be an important part of evaluating feasibility.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Thea DM, Steketee RW, Pliner V, Bornschlegel K, Brown T, Orloff S, et al. The effect of maternal viral load on the risk of perinatal transmission of HIV-1. New York City Perinatal HIV Transmission Collaborative Study Group. AIDS. 1997;11(4):437–44. https://doi.org/10.1097/00002030-199704000-00006.
Myer L, Phillips TK, McIntyre JA, Hsiao N-Y, Petro G, Zerbe A, et al. HIV viremia and mother-to-child transmission risk after antiretroviral therapy initiation in pregnancy in Cape Town. South Africa HIV Medicine. 2017;18:80–8. https://doi.org/10.1111/hiv.12397.
Nachega JB, Uthman OA, Anderson J, Peltzer K, Wampold S, Cotton MF, et al. Adherence to antiretroviral therapy during and after pregnancy in low-income, middle-income, and high-income countries: A systematic review and meta-analysis. AIDS. 2012;26(16):2039–52. https://doi.org/10.1097/QAD.0b013e328359590f.
Myer L, Dunning L, Lesosky M, Hsiao NY, Phillips T, Petro G, et al. Frequency of viremic episodes in HIV-infected women initiating antiretroviral therapy during pregnancy: A cohort study. Clin Infect Dis. 2017;64(4):422–7. https://doi.org/10.1093/cid/ciw792.
Haas AD, Msukwa MT, Egger M, Tenthani L, Tweya H, Jahn A, et al. Adherence to antiretroviral therapy during and after pregnancy: Cohort study on women receiving care in Malawi’s Option B+ program. Clin Infect Dis. 2016;63(9):1227–35. https://doi.org/10.1093/cid/ciw500.
Baryamutuma R, Kansiime E, Nuwagaba CK, Nabitaka L, Muhumuza S, Akello E, et al. An early assessment of Uganda’s roll-out of Option B+: Service capacity and infant outcomes. East Afr J Appl Health Monitor Eval. 2017;1:16–21.
Jain V, Owaraganise A, Black D, Twinamatsiko B, Ayebare M, Wandera B, et al. RAPID-VL intervention improves viral load ordering, results turnaround time, and viral suppression: A cluster randomized trial in HIV clinics in Uganda. 11th IAS Conference on HIV Science. 18–21 July, 2021. Virtual Event. Abstract # 2418.
Boeke CL, Joseph J, Atem C, Banda C, Coulibaly KD, Doi N, et al. Evaluation of near-POC viral load implementation in public health facilities across seven countries in sub-Saharan Africa. JIAS. 2020. https://doi.org/10.1002/jia2.25663.
Joseph J, Boeke CE, Makadzange EE, Sithole K, Maparo T, Mangwendeza PM, et al. Near POC VL testing during pregnancy and viremia at delivery. AIDS. 2022;36(5):711–9. https://doi.org/10.1097/QAD.0000000000003173.
Boyce RM, Ndizeye R, Ngelese H, Baguma E, Shem B, Rubinstein RJ, et al. “It takes more than a machine”: a pilot feasibility study of point-of-care HIV-1 viral load testing at a lower-level health center in rural western Uganda. PLoS Glob Public Health. 2023;3(3): e0001678. https://doi.org/10.1371/journal.pgph.0001678.
Drain PK, Dorward J, Violette LR, Quame-Amagio J, Thomas KK, Samsunder N. Point-of-care HIV viral load testing combined with task shifting to improve treatment outcomes (STREAM): Findings from an open-label, non-inferiority, randomized controlled trial. Lancet HIV. 2020;7(4):e229–37. https://doi.org/10.1016/S2352-3018(19)30402-3.
Moyo S, Mohammed T, Wirth KE, Prague M, Bennett K, Holme MP, et al. Point-of-Care Cepheid Xpert HIV-1 Viral Load Test in rural African communities is feasible and reliable. J Clin Microbiol. 2016;54:3050–5. https://doi.org/10.1128/JCM.01594-16.
Meggi B, Bollinger T, Zitha A, Mudenyanga C, Vubil A, Mutsaka D, et al. Performance of a true point-of-care assay for HIV-1/2 viral load measurement at antenatal and postpartum services. JAIDS. 2021;87(1):693–9. https://doi.org/10.1097/QAI.0000000000002621.
Uganda Ministry of Health. Consolidated Guidelines for the Prevention and Treatment of HIV and AIDS in Uganda. December 2016. https://www.prepwatch.org/wp-content/uploads/2017/08/consolidated_guidelines_hiv_prevention_uganda.pdf
Uganda Ministry of Health. Consolidated Guidelines for the Prevention and Treatment of HIV and AIDS in Uganda. November 2022. https://dsduganda.com/wp-content/uploads/2023/05/Consolidated-HIV-and-AIDS-Guidelines-20230516.pdf
Uganda AIDS Commission. 12th Annual Joint AIDS Review (JAR). FINAL REPORT July 2018-June 2019. 2019. https://www.unaids.org/sites/default/files/country/documents/UGA_2020_countryreport.pdf.
Kabami J, Balzer L, Kagoya F, Okiring J, Nangendo J, Arinitwe E, et al. Multicomponent intervention improves viral suppression for pregnant/postpartum women. 30th Conference on Retroviruses and Opportunistic Infections (CROI). February 19–22, 2023. Seattle, Washington, USA. Abstract #130.
Fairlee L, Sawry S, Pals S, Sherman G, Williamson D, Chivafa A. More frequent viral load testing, with point-of-care tests, has no impact on viral suppression in postpartum HIV-positive women in a randomized controlled trial in two clinics in Johannesburg. S Africa JIAS. 2021;24:S4. https://doi.org/10.1002/jia2.25755.
Kufa T, Mazanderani AH, Sherman GG, Mukendi A, Murray T, Moyo F, et al. Point-of-care HIV maternal viral load and early infant diagnosis testing around the time of delivery at tertiary obstetric units in South Africa: A prospective study of coverage, results return, and turn-around times. JIAS. 2020;23:e25487. https://doi.org/10.1002/jia2.25487.
Nakyanzi A, Naddunga F, Bulterys MA, Mujugira A, Wyatt MA, Kamusiime B, et al. “It soothes your heart”: A multi-method study exploring acceptability of point-of-care viral load testing among Ugandan pregnant and postpartum women living with HIV. Diagnostics (Basel). 2024;14(1):72. https://doi.org/10.3390/diagnostics14010072.
Kitebi Clinic. Kampala. Uganda: Unpublished Program Data; 2023.
Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–88. https://doi.org/10.1177/1049732305276687.
Rasti R, Nanjebe D, Karlstrom J, Muchunguzi C, Mwanga-Amumpaire J, Gantelius J, et al. Health care workers’ perceptions of point-of-care testing in a low-income country—a qualitative study in southwestern Uganda. PLoS ONE. 2017;12(7): e0182005. https://doi.org/10.1371/journal.pone.0182005.
Msimango L, Gibbs A, Shozi H, Ngobese H, Humphries H, Drain PK, et al. Acceptability of point-of-care viral load testing to facilitate differentiated care: A qualitative assessment of people living with HIV and nurses in South Africa. BMC Health Serv Res. 2020;20(1):1081. https://doi.org/10.1186/s12913-020-05940-w.
Qian RW, Hassan SA, Scallon AJ, Oyaro P, Brown E, Wagude J, et al. “After viral load testing, I get my results so I get to know which path my life is taking me”: Qualitative insights on routine centralized and point-of-care viral load testing in western Kenya from the Opt4Kids and Opt4Mamas Studies. BMC Health Serv Res. 2022;22:1540. https://doi.org/10.1186/s12913-022-08593-z.
Wiltsey Stirman S, Baumann AA, Miller CJ. The FRAME: An expanded framework for reporting adaptations and modifications to evidence-based interventions. Impl Sci. 2019;14:58. https://doi.org/10.1186/s13012-019-0898-y.
Acknowledgements
The authors gratefully acknowledge the Kingasa Study Team, who implemented the POC VL testing intervention, and the qualitative study participants, who took part in interviews. We also appreciate the contributions of the study clinic facility team, who were instrumental in implementing the Kingasa Study. We recognize the contributions of Paul Sendiwala, Grace K. Nalukwago, Collins C. Twesigye, and Jade Boyer. Finally, we thank Gwynn Stevens and Dipti Lallubhai for facilitating the donation of the Xpert instrument and Xpert HIV-1 Viral Load test kits.
Funding
This work was supported by a research grant to Dr. Celum from the US National Institutes of Mental Health [NIMH R01 MH113434].
Author information
Authors and Affiliations
Contributions
NCW and MAW designed the qualitative study. BK, AN(alumansi), and VK collected the qualitative data. EEP coordinated the data collection process and contributed to data analysis. CC conceptualized and designed the Kingasa Study. AN(akyanzi), FN, AM and CC implemented the Kingasa Study. NCW and MAW analyzed the qualitative data. NCW drafted the manuscript. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Kingasa Study was approved by the University of Washington Human Subjects Review Committee (STUDY00009286), the Mildmay Uganda Research Ethics Committee (MUREC 0707-2020), and the Uganda National Council for Science and Technology (HS991ES). All participants provided written informed consent to be interviewed as part of the overall study consent process.
Competing interests
Cepheid, Inc. donated the GeneXpert system and viral load test kits used in the Kingasa Study. AM reports a grant from Gilead Sciences, Inc., outside this work, and has served as advisor to ViiV Healthcare. CC has served as scientific advisor to Gilead Sciences and Merck.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ware, N.C., Wyatt, M.A., Nakyanzi, A. et al. POC viral load testing in an antenatal clinic setting for Ugandan pregnant women living with HIV: a qualitative implementation process analysis. Discov Health Systems 3, 44 (2024). https://doi.org/10.1007/s44250-024-00103-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44250-024-00103-8