Abstract
Background
Viral load (VL) monitoring of pregnant women living with HIV (PWLHIV) and antiretroviral therapy (ART) may contribute to lowering the risk of vertical transmission of HIV. The aims of this study were to assess the uptake of HIV VL testing among PWLHIV at entry to the prevention-of-mother-to-child transmission (PMTCT) services and identify facilitatory factors and barriers to HIV VL access.
Methods
A retrospective, cross-sectional study was conducted at 15 health facilities in Mutare district, Manicaland Province, Zimbabwe from January to December 2018. This analysis was complemented by prospective interviews with PWLHIV and health care providers between October 2019 and March 2020. Quantitative data were analysed using descriptive and inferential statistical methods. Risk factors were evaluated using multivariate logistic regression. Open-ended questions were analysed and recurring and shared experiences and perceptions of PWLHIV and health care providers identified.
Results
Among 383 PWLHIV, enrolled in antenatal care (ANC) and receiving ART, only 121 (31.6%) had a VL sample collected and 106 (88%) received their results. Among these 106 women, 93 (87.7%) had a VL < 1000 copies/mL and 77 (73%) a VL < 50 copies/mL. The overall median duration from ANC booking to VL sample collection was 87 (IQR, 7–215) days. The median time interval for the return of VL results from date of sample collection was 14 days (IQR, 7–30). There was no significant difference when this variable was stratified by time of ART initiation. VL samples were significantly less likely to be collected at local authority compared to government facilities (aOR = 0.28; 95% CI 0.16–0.48). Barriers to VL testing included staff shortages, non-availability of consumables and sub-optimal sample transportation. Turnaround time was prolonged by the manual results feedback system.
Conclusions and recommendation
The low rate of HIV VL testing among PWLHIV in Mutare district is a cause for concern. To reverse this situation, the Ministry of Health should consider interventions such as disseminating antiretroviral guidelines and policies electronically, conducting regular PMTCT mentorship for clinical staff members, and utilising point of care testing and telecommunication devices like mHealth to increase uptake of VL testing and improve results turnaround time.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
The human immunodeficiency virus (HIV) remains a leading cause of death among women during pregnancy and the postpartum period, especially in areas of high HIV prevalence [1]. Maternal plasma HIV viral load (VL) is the principal determinant of HIV vertical transmission [2,3,4,5,6]. Effective interventions during pregnancy, labour, delivery and breastfeeding reduces vertical transmission rates from 15 to 45% to below 5% [7].
There is no VL concentration above or below which vertical transmission of HIV always or never occurs [5, 8]. In published studies, the perinatal HIV transmission rate was significantly higher in pregnant women with VL concentrations of 50–400 copies/mL near delivery compared to concentrations < 50 copies/mL [9, 10]. Furthermore, among pregnant women living with HIV (PWLHIV) and receiving ART the risk of perinatal transmission of HIV is proportional to the maternal VL concentration [11]. Thus, while WHO uses a maternal VL of < 1000 copies/mL to define low risk of perinatal transmission of HIV, transmission of HIV may still occur below this VL concentration [12,13,14].
Since 2016 WHO guidelines have recommended that all PWLHIV should have an HIV VL test at 36 weeks gestational age to determine the risk status of infants and plan for their optimal management [7]. This intervention is meant to contribute to the reduction of vertical transmission and the elimination of mother to child transmission (eMTCT). Viral load monitoring during pregnancy can help guide the health care provider to institute more intensive adherence interventions if the pregnant woman is found to have a VL > 1000 copies/mL [3, 15]. Additionally, VL monitoring will aid the diagnosis and confirmation of ART failure [7]. Thus, VL monitoring plays an important role in the optimal management of prevention of mother to child transmission of HIV (PMTCT) interventions and is superior to immunological monitoring [16].
Zimbabwe, one of the high HIV burden countries in sub-Saharan Africa (SSA) with a prevalence of 14%, [17] adopted an amended version of the 2016 WHO guidelines [18]. Mutare district in Manicaland province started offering HIV VL testing in June 2015 with support from the Ministry of Health and Médecins Sans Frontières (MSF). By end of 2019, HIV VL services had been cascaded to 42 health facilities in Mutare district and included VL testing for pregnant and breastfeeding women. However, VL testing coverage in pregnant and breastfeeding women living with HIV has remained low at 63% at end of 2017 [19]. The aims of this study were to assess the uptake of HIV VL testing among PWLHIV at entry into the PMTCT services and identify facilitatory factors and barriers to HIV VL access at selected health facilities in Mutare district, Manicaland Province, Zimbabwe.
Methods
Study design
This cross-sectional study comprised two components, a retrospective descriptive analysis of demographic, clinical and VL data of PWLHIV who attended 15 health facilities in Mutare district between 1 January and 31 December 2018, sourced from ANC and ART registers, and patient booklets. Additionally, key informant interviews of PWLHIV at entry to the PMTCT services and health care providers were conducted prospectively at the same 15 health facilities in Mutare district between 1 October 2019 and 31 March 2020.
Study setting
Manicaland province is the largest of 10 provinces and situated in eastern Zimbabwe. It has a population of 1,862,000 with 983,000 being females, of whom 836,000 are women of childbearing potential [17]. The province has nine administrative districts, three of these (Mutare, Chipinge and Makoni) have rural and urban populations. The HIV prevalence in adults aged 15–49 years in the province is 10.5% [20].
Study sites
The 15 health facilities (study sites) were selected from 51 health facilities providing ANC services in Mutare district. The 51 health facilities were grouped by strata based on level of service provision and size i.e., clinic, rural/mission hospital, and tertiary hospital and geographical location, namely rural or urban location. The 15 study sites were then randomly selected by stratified sampling while ensuring representation by level of service provision and geographical location.
Sample size estimation
For the retrospective component, the sample size was estimated using a formula for a prevalence study, assuming 50% of PWLHIV at the 15 research sites received a VL test during their ANC visits, a confidence interval of 95% and study precision of 5%. The calculated sample size was 384. At each of the 15 study sites all PWLHIV who received ANC during the enrolment period (1 January to 31 December 2018) were listed sequentially in an excel spreadsheet according to their ANC numbers. The target number of PWLHIV for each site (Additional file 1: Table S1) was randomly selected. At sites where the target sample was equal to the number of PWLHIV who received ANC during the study period, all were included.
The structured prospective interviews using standardised questionnaires were conducted to provide more in-depth information from beneficiaries of ANC services and health care providers. The PWLHIV who were prospectively interviewed were a different group from those included in the retrospective analysis. For the prospective interviews there was no sample size estimation. Instead, the plan was to interview a minimum of one nursing professional at each of the 15 study sites, at least one PWLHIV per study site, and one laboratory scientist at the provincial laboratory. These individuals were selected by convenience sampling. For each of the study sites the prospective interviews were conducted on the same day that the retrospective data were abstracted.
Data collection
For the retrospective component, paper-based ANC and ART registers and patient booklets were reviewed at the 15 study sites and relevant data were extracted on paper-based datasheet and transferred to an electronic database in Epidata version 3.1 [21].
The responses of the prospectively interviewed PWLHIV and health care providers were manually entered in standardised questionnaire sheets and thereafter transferred to the electronic database in Epidata version 3.1 [21].
Data analysis
Quantitative data were exported to Stata version 15 (StataCorp, College Station, Texas, USA) for data cleaning and analysis [22]. Data were summarised using numbers and percentages for categorical variables, and medians and interquartile ranges for non-normally distributed continuous variables. The Wilcoxon rank-sum test was used to compare medians of non-normally distributed continuous variables. The Chi-squared test and Fischer’s exact test were used to compare categorical variables. Univariate- and multivariate-adjusted odds ratios [(a)OR] and their 95% confidence intervals (CIs) for factors associated with VL collection were explored using logistic regression. The multivariate logistic regression model was built, using variables which on univariate analysis had a p-value < 0.25. Parity and type of health facility were excluded from the logistic regression model because of their collinearity with gravidity and type of health facility ownership, respectively. Levels of significance were set at 5%. Structured questionnaires included open-ended questions that explored perceptions and experiences of VL testing. Open-ended questions were analysed, and recurring or shared experiences and perceptions identified. An independent reviewer (JS) supported the researcher’s (CCCM) interpretation of these responses.
Results
Study participants
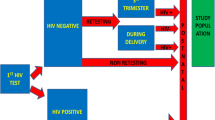
Data was obtained on 383 PWLHIV who were either on ART or ART naïve at the time of enrolment into ANC at the 15 study sites, representing 99.7% of the estimated sample size of 384 (Fig. 1 and Additional file 1: Table S1). Prospective interviews were completed by 12 nurses at 12 of the study sites, 1 laboratory scientist at the provincial.
Characteristics of study participants
The characteristics of the 383 women enrolled in the retrospective component of this study are summarised in Table 1. Nearly half of these women (46.7%) were aged 30–49 years. Only 42 (11.0%) of the PWLHIV presented for ANC booking during the first trimester. Among all PWLHIV with recorded ART status, 256 (66.8%) were already receiving ART by the time they were booked for ANC whilst 127 (33.1%) were newly tested HIV positive and started on ART. Most women were enrolled at primary health care facilities (82.5%) and most at health facilities belonging to a local authority (68.1%).
Viral load testing
Among the 383 PWLHIV in ANC and receiving ART, VL blood samples were collected for 121 (31.6%), of whom 106 (87.6%) received their VL results (Fig. 1). Among these 106 women, 93 (87.7%) had VL results of < 1000 copies/mL–77 had VL results < 50 copies/mL, 4 had VL results between 50 and 199 copies/mL and 11 had VL results between 200 and 999 copies/mL. Of the 9 women (8.5%) with VL results > 1000 copies/mL, three (33%) had follow-up VL tests done after enhanced adherence counselling (EAC). The follow-up VL results of all 3 women were < 1000 copies/mL.
The median duration between ANC booking and VL sample collection was significantly longer among those newly started on ART. However, the median duration for the return of VL results was not significantly different between the two groups (Table 2).
Factors associated with viral load sample collection
In the univariate analysis, receiving care at a rural hospital compared to a primary health care facility [OR = 2.94; 95% CI 1.45–5.96] was significantly associated with VL collection. Furthermore, VL samples were significantly less likely to be collected at local authority facilities compared to government facilities [OR = 0.28; 95% CI 0.17–0.46]. On multiple logistic regression the association of VL collection at local authority facilities compared to government facilities remained statistically significant [aOR = 0.28; 95% CI 0.16–0.48]. Gravidity, parity, gestational age, and ART status at ANC booking were not associated with VL sample collection (Table 3).
Structured interviews with pregnant women living with HIV
All 19 PWLHIV who participated in the prospective interviews reported that they had received information on VL testing during group health education talks provided before receiving ANC services, or during one-on-one consultation with a nurse or both. Twelve of the 19 (63.1%) women had a VL test done. Ten of 16 (62.5%) women who were interviewed understood what VL testing meant as substantiated by the following specific comments: Participant 10, a 42 year old woman diagnosed with HIV infection prior to her current pregnancy and already on ART stated that: “a viral load test detects the activity of the virus in one’s body”, while participant 18, a 29 year old woman with secondary education, presenting with her second pregnancy, newly diagnosed with HIV infection and started on ART stated that “a viral load test is taken to check if the ARVs I am taking are working and to check if I am responding to them”. However, four of the 16 (25%) women indicated that blood samples were taken but VL was not explained to them. The turnaround time (TAT) for VL results for ten women who responded ranged from 1 to 4 days. Thirteen of the 19 (68.4%) women understood the meaning of a low VL result; participant 14, a 29 year old woman having her second pregnancy stated that “a low VL result means that I am adhering to my treatment”; participant 11, a 32 year old woman pregnant for the third time, previously known to have HIV infection and on ART prior to her current pregnancy stated that “it means that the virus is being suppressed”; and participant 19, a 35 year old woman having her first pregnancy stated that “the virus strength is low, and my health is good”. Twelve of the 19 [63.1%] women understood the meaning of a high VL result.
Pregnant women living with HIV mentioned the following as factors that facilitated access to VL testing: VL sample collection synchronised with either ANC visits or antiretroviral (ARV) resupply days, free VL testing services, easy accessibility to the health facility and outreach services by nurses brought VL sample collection to their doorstep. The following barriers to accessing VL testing were cited by some PWLHIV: few nurses at the facility compared to the number of patients they serve meant that sometimes they deferred taking samples for VL testing, sometimes there were no supplies such as dry blood spot (DBS) consumables to enable the nurses to take the blood samples, some facilities were too far, hence PWLHIV could not access services on time, and some women could not access VL testing for religious reasons.
Interviews with health care providers
Of the 12 health care providers interviewed, 6 (50%) were primary care nurses (PCNs), 4 (33.3%) registered general nurses (RGNs) and 2 (16.7%) were midwives by training. Four of 12 health facilities (33.3%), all local authority health facilities, charged user fees for ANC services of one hundred Zimbabwean Dollars (equivalent to 5 United States Dollars) per visit. Laboratory services were free in all 12 facilities.
Ten of 12 (83.3%) health care providers were aware of the existence of the 2016 Zimbabwean ARV guidelines. Seven of the 12 health facilities had two or more nurses trained on the new guidelines. Training information was not available for the five remaining clinics. Ten (83,3%) nurses indicated that the guidelines were easy to understand. Although all twelve health care providers had received hard copies of the 2016 ARV guidelines it took up to 2 years for the guidelines to reach these facilities. Nine of 12 (75%) of the nurses were knowledgeable about the ARV guidelines, including the VL testing requirements. Eight of the 12 (66.7%) nurses provided feedback to their patients on their VL results during group education talks and the remaining four (33.3%) provided feedback during one-on-one consultations. Nine out of twelve (75%) facilities used dry blood samples (DBS). Only paper-based results were distributed to all 12 facilities. None of these facilities received their results via telephone or text messages. Viral load TAT was 1 to 2 weeks for six of the health facilities and between 2 and 8 weeks for the remaining six facilities.
Interview with the laboratory scientist
Viral load testing is centralised at the provincial laboratory staffed by 10 laboratory scientists and one laboratory technologist, all of whom were trained on VL testing. At the time of the interview, 225 facilities in the province submitted VL samples to the provincial laboratory of which 42 (18.7%) were in Mutare District. On average, 11,500 VL samples were received by the laboratory every month, of which 1% were from PWLHIV. According to the laboratory, TAT for results was on average 1–2 weeks from receipt of sample to release of results. The sample rejection rate was around 1%. Laboratory challenges in the provision of VL testing services included shortage of laboratory staff particularly data clerks, sample sorters and laboratory scientists, limited operating space, and delays in submitting whole blood specimens from some facilities. The laboratory information management system was not linked to the clinical services health information system at the facilities. Consequently, results were not available at health facility level in real time.
Health facility and patient related challenges
Health facility and patient related challenges faced from a health worker perspective in the provision of VL services are listed in Table 4.
Discussion
Antenatal care is the key health care entry point for pregnant women to access an array of health services for improving foetal and maternal outcomes [7]. WHO recommends that pregnant women have their first ANC contact within the first 12 weeks of gestation. In this study only 42 (11.0%) of PWLHIV presented for their ANC booking visits during the first trimester, lower than the findings of the Zimbabwe Demographic Health Survey (ZDHS, 2015/2016) which showed that 39% of PWLHIV and uninfected women booked in the first trimester. Another study completed in rural Mashonaland East province in 2017 showed that 29.2% of pregnant women booked during the first trimester, which is more than two-fold higher than this study, but still much lower than the national figure of 39% [23]. Late booking of PWLHIV implies that their HIV diagnosis and ART initiation could be delayed, increasing the risk of mother-to-child-transmission. A South African study observed that late ANC booking, and late ART initiation were associated with failure to achieve viral suppression in PWLHIV despite the services being free of charge. In this study delay in antenatal attendance was prevalent despite ANC and laboratory services being offered free of charge. Several studies in countries in the African region including South Africa [24, 25], Ethiopia [26] and Malawi [27] identified a spectrum of patient and health-related factors associated with late booking.
Among the PWLHIV enrolled into ANC in this study, only 31.6% had a VL test done. Although this coverage is comparable to studies done in the East and Southern African region, this is unacceptably low given that Manicaland province was among the first provinces in Zimbabwe to be supported to provide VL testing. Viral load testing in Manicaland province at the commencement of this service was initially focussed on pregnant and breastfeeding women, children and general clients with treatment failure then expanding to routine VL testing [28,29,30,31]. Viral load coverage at national level was higher at 44% and 54% in 2018 and 2019 respectively [32]. Another study in Zimbabwe recorded a VL testing coverage of 31.9% in a cohort of pregnant and breastfeeding women [33]. Viral load coverage was 63.1%, almost two-fold higher in 16 women interviewed for this study between October 2019 and March 2020. Although this coverage was higher than the 31.6% recorded in the retrospective component completed in 2018 the sample size was very small. Thus, further study of a larger, more representative sample is needed to determine whether VL testing coverage in pregnant women on ART has increased over time.
Women who were already on ART at the time of ANC booking had their VL tests done much earlier than the women who were newly started on ART. This was aligned to the Zimbabwean guidelines which stated that women who are already on ART would have a VL test done at first ANC booking, while for newly initiated women, VL testing was to be conducted 3 months after the first ANC booking. Of concern was that the median time from booking to having a VL test done for newly initiated women was 207 days meaning that these women had their VL test done a median of 6.9 months after ART initiation instead of the recommended 3 months after ART initiation.
The median TAT between VL specimen collection and receipt of VL results by the PWLHIV for this study was 14 days (IQR, 7–30); this is still unacceptably high though it compared well to the maximum TAT target for the programme of 14 days [34]. Point of care testing could shorten the TAT by providing same day results, thereby benefiting VL unsuppressed women as they can get their results during the same clinic visit and be managed appropriately. A study in Botswana concluded that use of point of care (POC) testing is a feasible and reliable option for VL monitoring, even in rural settings and that this would provide same-day VL results, allow for immediate assessment of virologic failure and reduce loss to follow-up [35].
Nicholas et al. [36] evaluated the outcomes of the first 4 years of routine VL monitoring using POC in rural clinics in Malawi and concluded that high VL coverage can be achieved followed by same-day test results and shorter time-to-switch to new ARV regimens. The game-changing potential of POC-based VL testing compared to conventional testing was also demonstrated [37, 38]. Our study showed that the major challenges were unreliable transport for specimens to the laboratory and long TAT. These challenges can be solved with the introduction and scale up of POC technology [39].
Of the PWLHIV who had VL testing, 87.6% received their results and of these 87.7% had a VL < 1000 copies/mL. This is a good outcome and indicates good adherence to ARVs among pregnant women living with HIV which can lead to reduction in MTCT. In our study of 106 who had VL testing 77 (72.6%) were virally suppressed to < 50 copies/mL. Similar high VL suppression rates (< 50 copies/mL) were documented in studies in Rwanda [40], Ethiopia [41, 42] and Nigeria [43].
Our results showed that VL specimens were less likely to be collected at local authority facilities compared to government facilities. This observation requires more research to establish the actual reasons for the difference in testing practices. A similar study by Atuhaire et al. [44] in 2015 among PWLHIV at five local authority health facilities also noted a difference in practices in VL testing among the facilities, however it was noted that more research was required to establish the cause of such differences. Lessons learned from the Therapeutic Solidarity and Initiatives against AIDS, OPP-ERA project in five West African countries showed that disparities in VL sample collection can be due to multiple determinants including the number, training and availability of health staff, size of the HIV cohort at the facility, and organisation of the sample collection circuit [45]. In that study enabling factors for VL sample collection included the provision of a one stop shop for HIV services or the supermarket approach, where the pregnant women receive all their services under one roof from the same health worker and on the same day, and the provision of free ANC services. Ease of collection and preparation of DBS versus whole blood sample collection also facilitated the collection VL samples. Age, gravidity, parity, and gestational age at ANC booking were not predictors for VL sample collection. The barriers affecting VL collection were mostly systemic and included staff shortages, non-availability of consumables and laboratory forms and weaknesses in sample transportation. Lecher et al. [46] noted similar challenges during monitoring of progress with scale-up of HIV VL in seven sub-Saharan African countries.
Findings from the interviews of nursing personnel indicated that they were aware of the national guidelines on VL testing in pregnant women. Despite possessing this knowledge, VL testing coverage was low. It was noted in a retrospective cohort analysis of patients eligible for routine VL testing between 2013 and 2017 in Malawi and a retrospective data review of routine reports from MSF-supported health facilities in Zimbabwe that despite health care providers knowledge of the guidelines, there is need for regular staff training, mentorship and motivation, and continuous monitoring of the implementation of guidelines. Enablers such as reliable sample and results transport systems, quality-assured testing laboratories and demand creation are essential to the success of routine VL testing scale up. In our study it was also revealed that paper-based results were sent to facilities using motorbikes. In this technological era, use of mHealth to provide same-day results through SMS technology will speed up the TAT and address the issue of unreliable transport to deliver results to facilities.
The delay in guideline dissemination results in delay in implementation of the policies with the result that some pregnant women living with HIV did not receive the preferred services. Being knowledgeable on a subject or policy does not necessarily translate to implementation of guidelines as there are other factors that contribute to successful implementation [36, 47].
Women who were interviewed indicated that they were provided with or received information during group education talks or during consultation. However, the women interviewed felt that the health education talks given while awaiting ANC were too brief and there was insufficient time for pregnant women to ask questions. In addition, though they appreciated the one-on-one consultation, they were still not given enough time to ask questions and discuss HIV-related issues including VL testing. Consultations were constrained by high patient volume. Van Bogaert et al. [48] reported similar findings whereby nurses indicated that information about diagnostics and treatment were brief, and patients’ questions and worries were neglected when the workload was heavy.
One of the best suggestions for improving VL testing practice that emerged from the nurse interviews was that VL sample collection be synchronised with either ANC visits or ARV resupply days. Some facilities provided outreach services in hard-to-reach areas, thereby improving access to care for some pregnant women living with HIV. Outreach services is one of the recognized differentiated service delivery models in provision of HIV services [49, 50].
Strengths and limitations of this study
This descriptive cross-sectional study utilised collected facility-level data that reflects routine clinical practice [51]. Although the retrospective component used an adequately powered, well represented sample, the prospective interviews were conducted on small samples. Additionally, the interviews of PWLHIV were limited to 10 of the 15 research sites. Thus, the findings may not be truly representative of the health worker population and population of pregnant women living with HIV in Manicaland province.
Data deficits were also cited by the nurses and observed by the researchers during data abstraction and these data gaps from both paper-based registries and the electronic systems were attributed as a major contributor to the low VL testing coverages. Additionally, due to the retrospective design there were limitations in the availability and completeness of demographic and clinical data. Gaps in routine data has been well documented in studies that have utilised retrospective data sources [33, 36, 40], and these limitations impacted the quality of the data for such studies [19, 36, 40, 52, 53].
The cross-sectional design did not allow description of trends over time including the impact of the coronavirus disease 2019 pandemic on VL testing. Whether VL testing increased after 2018 as suggested by the response of 16 pregnant women living with HIV during the interviews could not be confirmed. Further study is needed to determine whether VL testing, and factors associated with VL testing have changed over time.
Conclusions
The low rate of HIV VL testing among PWLHIV in Mutare district is cause for concern requiring urgent attention. The Ministry of Health should consider disseminating ARV guidelines/policy documents electronically which is quicker and may translate into faster implementation of new policies. There is need to conduct regular mentorship and establish quality improvement initiatives for PMTCT services. Alerts within the ePMS or on the files of PWLHIV may ensure that VL testing is done on time so that missed testing opportunities are minimised particularly in newly identified pregnant women living with HIV. Point of care technology and mHealth can reduce VL results TAT. Recommendations from the study can potentially contribute to improved provision of VL testing services among PWLHIV in Mutare district, the province and nationally.
Availability of data and materials
The dataset used and/or analysed in this study is available from the corresponding author on reasonable request.
Abbreviations
- AIDS:
-
Acquired Immunodeficiency syndrome
- ANC:
-
Antenatal care
- ART:
-
Antiretroviral therapy
- ARV:
-
Antiretroviral
- aOR:
-
Adjusted odds ratio
- CI:
-
Confidence interval
- EDTA:
-
Ethylenediamine tetra acetic acid
- eMTCT:
-
Elimination of mother to child transmission
- ePMS:
-
Electronic patient monitoring system
- IQR:
-
Interquartile range
- HIV:
-
Human Immunodeficiency Virus
- mHealth:
-
Mobile health
- MSF:
-
Médecins Sans Frontières
- MTCT:
-
Mother to child transmission
- OR:
-
Odds ratio
- OPP-ERA:
-
Open polyvalent platforms (OPP) for sustainable and quality access to VL in resource limited settings
- PWLHIV:
-
Pregnant women living with HIV
- PCN:
-
Primary care nurse
- POC:
-
Point of care
- RGN:
-
Registered general nurse
- TAT:
-
Turnaround time
- UCT:
-
University of cape town
- UNICEF:
-
United Nations Children’s Fund
- UNAIDS:
-
Joint United Nations Programme on HIV & AIDS
- VL:
-
Viral load
- WHO:
-
World Health Organisation
- ZDHS:
-
Zimbabwe demographic health survey
- ZIMPHIA:
-
Zimbabwe population-based HIV impact assessment
References
World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection recommendations for a public health approach. Geneva: World Health Organization; 2016.
Tubiana R, Le Chenadec J, Rouzioux C, Mandelbrot L, Hamrene K, Dollfus C, et al. Factors associated with mother-to-child transmission of HIV-1 despite a maternal viral load < 500 copies/ml at delivery: a case-control study nested in the French perinatal cohort (EPF-ANRS CO1). Clin Infect Dis. 2010;50(4):585–96.
Myer L, Essajee S, Broyles LN, Watts DH, Lesosky M, El-Sadr WM, et al. Pregnant and breastfeeding women: a priority population for HIV viral load monitoring. PLoS Med. 2017;14:8.
Chagomerana MB, Miller WC, Tang JH, Hoffman IF, Harrington BJ, DiPrete B, et al. Prevalence of antiretroviral therapy treatment failure among HIV-infected pregnant women at first antenatal care: PMTCT option B+ in Malawi. PLoS ONE. 2018;13:12.
Garcia PM, Kalish LA, Pitt J, Minkoff H, Quinn TC, Burchett SK, et al. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. N Engl J Med. 1999;341(6):394–402.
Ahmed S, Kim MH, Abrams EJ. Risks and benefits of lifelong antiretroviral treatment for pregnant and breastfeeding women: a review of the evidence for the option B+ approach. Curr Opin HIV AIDS. 2013;8(5):474–89.
World Health Organization. Progress report 2016: prevent HIV, test and treat all: WHO support for country impact. Geneva: World Health Organization; 2016.
Ioannidis JP, Abrams EJ, Ammann A, Bulterys M, Goedert JJ, Gray L, et al. Perinatal transmission of human immunodeficiency virus type 1 by pregnant women with RNA virus loads < 1000 copies/ml. J Infect Dis. 2001;183(4):539–45.
Hermans LE, Carmona S, Nijhuis M, Tempelman HA, Richman DD, Moorhouse M, et al. Virological suppression and clinical management in response to viremia in South African HIV treatment program: a multicenter cohort study. PLoS Med. 2020;17(2):e1003037.
Joya C, Won SH, Schofield C, Lalani T, Maves RC, Kronmann K, et al. Persistent low-level viremia while on antiretroviral therapy is an independent risk factor for virologic failure. Clin Infect Dis. 2019;69(12):2145–52.
Mandelbrot L, Tubiana R, Le Chenadec J, Dollfus C, Faye A, Pannier E, et al. No perinatal HIV-1 transmission from women with effective antiretroviral therapy starting before conception. Clin Infect Dis. 2015;61(11):1715–25.
Puthanakit T, Thepnarong N, Chaithongwongwatthana S, Anugulruengkitt S, Anunsittichai O, Theerawit T, et al. Intensification of antiretroviral treatment with raltegravir for pregnant women living with HIV at high risk of vertical transmission. J Virus Erad. 2018;4(2):61.
Kuhn L, Strehlau R, Shiau S, Patel F, Shen Y, Technau K-G, et al. Early antiretroviral treatment of infants to attain HIV remission. E Clin Med. 2020;18:100241.
Faye A. Early antiretroviral treatment of infants to attain HIV remission: not just a matter of timing. E Clin Med. 2020. https://doi.org/10.1016/j.eclinm.2020.100284.
Roberts T, Cohn J, Bonner K, Hargreaves S. Scale-up of routine viral load testing in resource-poor settings: current and future implementation challenges. Clin Infect Dis. 2016;62(8):1043–8.
Sigaloff KC, Hamers RL, Wallis CL, Kityo C, Siwale M, Ive P, et al. Unnecessary antiretroviral treatment switches and accumulation of HIV resistance mutations; two arguments for viral load monitoring in Africa. J Acquir Immune Defic Syndr. 2011;58(1):23–31.
Agency ZNS. Inter-censal demographic survey, 2017. Zimbabwe: Zimstats Harare; 2017.
Ministry of Health and Child Care (MOHCC). 2016 Guidelines for antiretroviral therapy for the prevention and treatment of HIV in Zimbabwe, 2016. In: The National Medicine and Therapeutics Policy Advisory Committee (NMTPAC) and The AIDS and TB directorate, editor. Harare.
Nyagadza B, Kudya N, Mbofana E, Masaka S, Garone D, Chen C, et al. Scaling up HIV viral load monitoring in Manicaland, Zimbabwe: challenges and opportunities from the field. Public Health Action. 2019;9(4):177–81.
Zimbabwe National Statistics Agency and ICF International. 2018 Zimbabwe demographic and health survey 2015: Final Report. Rockville: Zimbabwe National Statistics Agency (ZIMSTAT) and ICF international 2016. Addis Ababa. Ethiopia
Christiansen T, Lauritsen J. EpiData-comprehensive data management and basic statistical analysis system. Odense: Epi Data Assoc; 2010.
StataCorp. Stata statistical software: release 17. College Station TX: Stata Corp LLC; 2015.
Mhlanga M, Zvinavashe M, Gwanzura L, Stray-Pedersen B. Factors associated with maternal and child health services uptake and their association with health outcomes in Mashonaland East Zimbabwe. Clin Mother Child Heal. 2017;14:3.
Ebonwu J, Mumbauer A, Uys M, Wainberg ML, Medina-Marino A. Determinants of late antenatal care presentation in rural and peri-urban communities in South Africa: a cross-sectional study. PLoS ONE. 2018;13:3.
Kaswa R, Rupesinghe GF, Longo-Mbenza B. Exploring the pregnant women’s perspective of late booking of antenatal care services at Mbekweni Health Centre in Eastern Cape, South Africa. Afri J Prim Health Care Fam Med. 2018;10(1):1–9.
Grum T, Brhane E. Magnitude and factors associated with late antenatal care booking on first visit among pregnant women in public health centers in central zone of Tigray Region, Ethiopia: a cross sectional study. PLoS ONE. 2018;13:12.
Manda-Taylor L, Sealy D, Roberts J. Factors associated with delayed Antenatal care attendance in Malawi: Results from a qualitative study. Med J Zambia. 2017;44(1):17–25.
Komtenza B, Satyanarayana S, Takarinda KC, Mukungunugwa SH, Mugurungi O, Chonzi P, et al. Identifying high or low risk of mother to child transmission of HIV: how Harare City, Zimbabwe is doing? PLoS ONE. 2019;14:3.
Kubheka SE, Archary M, Naidu KK. HIV viral load testing coverage and timeliness after implementation of the wellness anniversary in a paediatric and adolescent HIV clinic in KwaZulu-Natal, South Africa. Southern Afr J HIV Med. 2020;21(1):1–5.
Maheu-Giroux M, Marsh K, Doyle CM, Godin A, Delaunay CL, Johnson LF, et al. 2019 National HIV testing and diagnosis coverage in sub-Saharan Africa: a new modeling tool for estimating the ‘first 90’from program and survey data. AIDS 2009;33 (Suppl 3):S255-S269
Peter T, Zeh C, Katz Z, Elbireer A, Alemayehu B, Vojnov L, et al. Scaling up HIV viral load–lessons from the large-scale implementation of HIV early infant diagnosis and CD 4 testing. J Int AIDS Soc. 2017;20:e25008.
Ministry of Health and Child Care (MOHCC). 2018 Zimbabwe health sector performance monitoring and evaluation policy guidelines and strategy.
Mahachi N, Muchedzi A, Tafuma TA, Mawora P, Kariuki L, Bw S, et al. Sustained high HIV case-finding through index testing and partner notification services: experiences from three provinces in Zimbabwe. J Int AIDS Soc. 2019;22:e25321.
Ministry of Health and Child Care (MOHCC). ZIMBABWE HIV viral load scale-up plan 2015–2018. Zimbabwe: Harare; 2015.
Moyo S, Mohammed T, Wirth KE, Prague M, Bennett K, Holme MP, et al. Point-of-care cepheid Xpert HIV-1 viral load test in rural African communities is feasible and reliable. J Clin Microbiol. 2016;54(12):3050–5.
Nicholas S, Poulet E, Wolters L, Wapling J, Rakesh A, Amoros I, et al. Point-of-care viral load monitoring: outcomes from a decentralized HIV programme in Malawi. J Int AIDS Soc. 2019;22(8):e25387.
Bianchi F, Cohn J, Sacks E, Bailey R, Lemaire J-F, Machekano R, et al. Evaluation of a routine point-of-care intervention for early infant diagnosis of HIV: an observational study in eight African countries. Lancet HIV. 2019;6(6):e373–81.
Mwenda R, Fong Y, Magombo T, Saka E, Midiani D, Mwase C, et al. Significant patient impact observed upon implementation of point-of-care early infant diagnosis technologies in an observational study in Malawi. Clin Infect Dis. 2018;67(5):701–7.
Nyakura J, Shewade HD, Ade S, Mushavi A, Mukungunugwa SH, Chimwaza A, et al. Viral load testing among women on ‘option B+’in Mazowe, Zimbabwe: how well are we doing? PLoS ONE. 2019;14:12.
Gill MM, Hoffman HJ, Bobrow EA, Mugwaneza P, Ndatimana D, Ndayisaba GF, et al. Detectable viral load in late pregnancy among women in the Rwanda option B+ PMTCT program: enrollment results from the Kabeho study. PLoS ONE. 2016;11:12.
Demissie DB, Bulto GA, Mekuria WT, Dufera FN, Gamshe EN. Evaluation of antiretroviral therapy initiated among pregnant women under option B+ by viral load and CD4 count outcomes in selected hospitals of west shewa zone, oromia region, Ethiopia. HIV/AIDS. 2020;12:127.
Tolossa T, Kassa GM, Chanie H, Abajobir A, Mulisa D. Incidence and predictors of lost to follow-up among women under option B+ PMTCT program in western Ethiopia: a retrospective follow-up study. BMC Res Notes. 2020;13(1):18.
Omonaiye O, Kusljic S, Nicholson P, Mohebbi M, Manias E. Post Option B+ implementation programme in Nigeria: determinants of adherence of antiretroviral therapy among pregnant women with HIV. Int J Infect Dis. 2019;81:225–30.
Atuhaire P, Brummel SS, Mmbaga BT, Angelidou K, Fairlie L, Violari A, et al. The impact of short term antiretroviral therapy (ART) interruptions on longer term maternal health outcomes—a randomized clinical trial. PLoS ONE. 2020;15(1):e0228003.
Solthis. 2019 HIV viral load testing operational guide 60 lessons learned from the open polyvalent platforms (OPP) for sustainable and quality access to vl in resource limited settings (OPP-ERA) Project
Lecher S, Williams J, Fonjungo PN, Kim AA, Ellenberger D, Zhang G, et al. Progress with scale-up of HIV viral load monitoring—seven sub-Saharan African countries, january 2015–june 2016. Morb Mortal Wkly Rep. 2016;65(47):1332–5.
Mutabazi JC, Zarowsky C, Trottier H. The impact of programs for prevention of mother-to-child transmission of HIV on health care services and systems in sub-Saharan Africa-a review. Public Health Rev. 2017;38(1):28.
Van Bogaert P, Peremans L, Van Heusden D, Verspuy M, Kureckova V, Van de Cruys Z, et al. Predictors of burnout, work engagement and nurse reported job outcomes and quality of care: a mixed method study. BMC Nurs. 2017;16(1):5.
Trafford Z, Gomba Y, Colvin CJ, Iyun VO, Phillips TK, Brittain K, et al. Experiences of HIV-positive postpartum women and health workers involved with community-based antiretroviral therapy adherence clubs in Cape Town, South Africa. BMC Public Health. 2018;18(1):935.
Vrazo AC, Sullivan D, Phelps BR. Eliminating mother-to-child transmission of HIV by 2030: 5 strategies to ensure continued progress. Glob Health Sci Pract. 2018;6(2):249–56.
Regnault A, Willgoss T, Barbic S. Research ISfQoL towards the use of mixed methods inquiry as best practice in health outcomes research. J Patient Rep Outcomes. 2018;2(1):19.
Anderson C, Low-Choy S, Whittle P, Taylor S, Gambley C, Smith L, et al. Australian plant biosecurity surveillance systems. Crop Prot. 2017;100:8–20.
Ntlantsana V, Hift RJ, Mphatswe WP. HIV viraemia during pregnancy in women receiving preconception antiretroviral therapy in KwaDukuza, KwaZulu-Natal. South Afr J HIV Med. 2019;20(1):1–8.
Acknowledgements
The authors wish to thank Nomalanga Marimo (field coordinator), Joylyn Chatora and Doreen Saganya (research assistants) for their contribution to the research. We acknowledge the Permanent Secretary, Director of HIV, AIDS, STI, Hepatitis and TB Programmes, PMTCT Programme Manager Ministry of Health and Child Care, Zimbabwe, Medical Director and HIV/STI Focal Person, Manicaland Province and the District Medical Officer, Mutare District for permission to conduct the research; and we wish to thank the District Health Team, the staff at the study facilities and the pregnant women living with HIV who agreed to participate in the study and share their experiences.
Funding
CCM provided funding for the study.
Author information
Authors and Affiliations
Contributions
CCM conceptualised and carried out the study and wrote the manuscript. KCT conducted the statistical analysis. BE, JW, and IC contributed to the study protocol, guided the statistical analysis, and contributed to the development and revision of the of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
The study protocol was approved by the Medical Research Council of Zimbabwe (reference number: MRCZ/B/1732) and the Human Research Ethics Committee (HREC) Faculty of Health Sciences, University of Cape Town, (reference number: HREC REF:778/2018). Administrative approval for the study was obtained from the Ministry of Health and Child Care, AIDS, and TB Programme and from the Provincial and District Directorates. Informed written consent was obtained from health care providers and all pregnant women living with HIV who participated in the prospective interviews for this study. Data was deidentified during abstraction and anonymously analysed. No financial incentives were provided to the study participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Table 1 Distribution of participants at the fifteen study sites in Mutare District, Zimbabwe
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Musanhu, C.C.C., Takarinda, K.C., Shea, J. et al. Viral load testing among pregnant women living with HIV in Mutare district of Manicaland province, Zimbabwe. AIDS Res Ther 19, 52 (2022). https://doi.org/10.1186/s12981-022-00480-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12981-022-00480-1