Abstract
In recent decades, various bioanalytical technologies have been investigated for appropriate medical treatment and effective therapy. Temperature-responsive chromatography is a promising bioanalytical technology owing to its functional properties. Temperature-responsive chromatography uses a poly(N-isopropylacrylamide)(PNIPAAm) modified stationary phase as the column packing material. The hydrophobic interactions between PNIPAAm and the analyte could be modulated by changing the column temperature because of the temperature-responsive hydrophobicity of PNIPAAm. Thus, the chromatography system does not require organic solvents in the mobile phase, making it suitable for therapeutic drug monitoring in medical settings such as hospitals. This review summarizes recent developments in temperature-responsive chromatography systems for therapeutic drug monitoring applications. In addition, separation methods for antibody drugs using PNIPAAm are also summarized because these methods apply to the therapeutic drug monitoring of biopharmaceutics. The temperature-responsive chromatography systems can also be utilized for clinical diagnosis, as they can assess multiple medicines simultaneously. This highlights the significant potential of temperature-responsive chromatography in medicine and healthcare.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent decades, biomedical analysis has increased in importance in the clinical field because analyzing drugs, biomarkers, and bioactive concentrations in the living body is important for appropriate medical treatment and effective therapy [1,2,3,4,5]. Therapeutic drug monitoring (TDM) is essential for biomedical analysis. TDM is the method for measuring drug concentration in serum and is an effective approach to monitor the therapeutic effect using specific types of drugs [6, 7]. The appropriate dosage of certain medications must be modified by assessing the concentration of the drug in the blood serum, as an insufficient concentration of the drug will prove ineffective, whereas an excessively high concentration can lead to toxic side effects. Thus, TDM is required to monitor the drug concentration.
Liquid chromatography (LC) is a reliable method for measuring drug concentration in serum because of its adaptability and the absence of a specific antibody for each individual drug [8]. However, most of LC requires the presence of organic solvents in the mobile phase to regulate drug retention in the chromatography column. Furthermore, it is necessary to deproteinate the blood samples using organic solvents before determining the drug concentration in the serum using chromatography. Organic solvents are commonly prohibited in hospitals owing to their potential hazards. Therefore, a technique that does not require organic solvents is desirable for TDM. Chromatography, which utilizes poly(N-isopropylacrylamide) (PNIPAAm) to respond to temperature changes is a measurement technique, and it does not employ organic solvents in mobile phase [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29] (Fig. 1).
PNIPAAm is a thermoresponsive polymer whose hydrophobicity changes in response to temperature changes (Fig. 1A). The hydrophilic and hydrophobic properties of PNIPAAm, which are influenced by temperature, change as a result of hydration and dehydration at a lower critical solution temperature (LCST) of 32 °C [30,31,32,33,34,35,36]. Also, the PNIPAAm exhibits temperature-modulated extension and shrinking attributed to the hydration and dehydration. This thermoresponsive polymer has been widely utilized in biomedical applications because of its relatively low LCST, which is close to the human body temperature. Examples of these applications include temperature-controlled drug and gene delivery systems [37,38,39,40,41,42,43,44], biosensors and bioimaging systems [45,46,47,48,49,50,51,52], nanoactutors [53,54,55,56,57,58,59], bioseparation tools [60,61,62,63,64,65,66,67,68,69,70,71,72,73,74], cell separation materials [75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90], and cell culture substrates for tissue engineering [91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110].
The ability of PNIPAAm to change its properties in response to temperature has been harnessed for the development of bioanalysis systems such as temperature-responsive chromatography (Fig. 1B). In this technique, the packing beads of the chromatography columns were modified with PNIPAAm, and the interactions between the material and target drugs were adjusted by altering the column temperature.
In an ordinary chromatography system using octadecylsilyl (ODS) group-modified bead-packed columns, hydrophobic interactions between analytes and ODS groups are modulated by the addition of an organic solvent to the mobile phase. By contrast, the PNIPAAm-modified stationary phases were used in temperature-responsive chromatography (Fig. 1B). Because the hydrophobic properties of PNIPAAm can be influenced by external temperature changes owing to hydration and dehydration of the polymer, the surface properties of the PNIPAAm-modified stationary phase can be easily adjusted by altering the column temperature. This allows for the modulation of the hydrophobic interactions between the analytes and the stationary phase. Therefore, retention modulation is quite simple compared to the adjustment of the mobile phase composition (Fig. 1C). The chromatographic system does not require the addition of an organic solvent to the mobile phase and allows for analysis using an all-aqueous mobile phase. Chromatography systems prevent exposure to organic solvents in hospitals, making them suitable for TDM.
This review describes recent developments in TDM techniques using PNIPAAm and their properties. In addition, separation methods for antibody drugs using PNIPAAm, which are applicable for TDM, are also summarized.
TDM using PNIPAAm modified beads packed chromatography column
HPLC columns using PNIPAAm-modified beads have been developed as innovative chromatography methods because the chromatography system does not require the addition of an organic solvent to the mobile phase, and analyte retention can be modulated by simply changing the column temperature. Therefore, chromatography columns have been investigated for applications in TDM because an all-aqueous mobile phase (without organic solvents) is suitable for use in medical settings.
A temperature-responsive chromatography column with thin PNIPAAm hydrogel layer-modified silica beads was used for TDM (Fig. 2) [111]. PNIPAAm hydrogel modification was performed using the radical initiator V-501, followed by radical polymerization of NIPAAm and methylene bis-acrylamide (BIS) (Fig. 2A). The feasibility of the TDM column was investigated using phenytoin, lamotrigine, carbamazepine, disopyramide, quinidine, propafenone, digoxin, vancomycin, mycophenolic acid, and methotrexate as TDM drugs. The elution behavior of each type of drugs containing serum as a contaminant was observed. In the chromatogram, all serum proteins and drugs were separated and eluted as two peaks with short analysis times (Fig. 2C). Serum was immediately eluted, and drug was slightly retained on the column because the serum proteins did not effectively interact with PNIPAAm and most of the drugs interacted with PNIPAAm through hydrophobic interactions. These results indicate that TDM can be performed using a PNIPAAm-modified bead-packed column with all aqueous mobile phases.
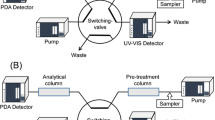
Serum proteins in drug sample often adsorb the stationary phase of HPLC columns leading to the reduced function of the column and reproducibility of the drug concentration measurement. To resolve the problem, temperature-responsive chromatography was applied to a two-dimensional HPLC system (Fig. 3) [112]. Primary column was used to separate the serum protein and drug, and serum protein was flowed out and drug was introduced to the second column. The secondary column was used to determine the drug concentration.
Two-dimensional temperature-responsive chromatography for TDM. (A) Preparation scheme for dilute PNIPAAm brush-modified silica beads, (B) Temperature-modulated interaction between PNIPAAm and analytes, (C) Conceptual diagram of two-dimensional temperature-responsive chromatography for TDM, and (D) Chromatograms of the drug-serum samples with column switching
Dilute PNIPAAm brush-modified silica beads were prepared using atom transfer radical polymerization (Fig. 3A). The density of PNIPAAm was reduced by introducing 3-glycidyloxypropyltrimethoxysilane (GPTMS) during ATRP initiator modification of the silica beads. The diluted density of PNIPAAm on the silica beads shortens the retention time of the analyte compared to the dense PNIPAAm brush, which is attributed to reduced hydrophobic interactions between PNIPAAm and the analyte [26]. The prepared columns were used in a two-dimensional HPLC system (Fig. 3C). The primary column was used to separate the serum proteins and drugs. After elution of the serum protein, the drug was introduced into the second column, and the drug concentration was determined. The elution behaviors of 13 drugs (carbamazepine, lamotrigine, zonisamide, phenobarbital, nitrazepam, diazepam, disopyramide, quinidine, propafenone, sotalol, voriconazole, lidocaine, and theophylline) were observed using two-dimensional temperature-responsive chromatography. Using a two-dimensional temperature-responsive chromatography system, drug peaks without serum peaks were observed in the secondary column (Fig. 3D). The quantitative determination of drugs in serum proteins can be performed using the chromatogram of the secondary column. The results indicated that the two-dimensional temperature-responsive chromatography system could remove serum proteins from the drug through the primary column, and precise quantitative determination was performed using a secondary column.
TDM using temperature-responsive ion exchange chromatography
As various types of drugs have ionic properties, ion-exchange chromatography is suitable for their effective retention. Thus, temperature-responsive ion-exchange chromatography was investigated for TDM [113, 114].
Temperature-responsive anion-exchange chromatography was developed to effectively interact with anionic drugs (Fig. 4) [113].
Temperature-responsive ion-exchange chromatography for TDM. (A) Preparation scheme for cationic copolymer-modified silica beads, (B) Temperature-modulated interactions between copolymer and analytes, (C) Conceptual diagram of cationic property of the polymer-modified silica beads, and (D) Chromatograms of drug sample on the prepared column
The cationic monomer N,N-dimethylaminopropyl acrylamide (DMAPAAm), was introduced into PNIPAAm on silica beads via copolymerization (Fig. 4A). n-Butylmethacrylate (BMA) was introduced to the polymer to modulate its hydrophobicity. The cationic properties of the polymers were modulated by varying the amount of DMAPAAm added. The 13 drugs, disopyramide, voriconazole, lidocaine, zonisamide, mexiletine, carbamazepine, quinidine, phenytoin, lamotrigine, diazepam, phenobarbital, sotalol, and mycophenolic acid, were used as model analytes, and the elution behavior of the drugs from the copolymer modified beads packed column was observed. Phenobarbital and lamotrigine were effectively retained on the strongly cationic polymer-modified bead-packed column (DMAPAAm3%) (Fig. 4D). Sotalol, quinidine, and mycophenolic acid effectively retained the weakly cationic polymer-modified bead-packed column (DMAPAAm1%). Voriconazole, zonisamide, diazepam, phenytoin, carbamazepine, mexiletine, lidocaine, and disopyramide were effectively retained on a noncationic polymer bead-packed column (DMAPAAm0%). This is attributed to the suitable balance between the hydrophobic and cationic interactions between the polymer and drugs. With an increase in the amount of cationic monomers in the polymer, the cationic properties improved. By contrast, with an increase in the amount of cationic monomers in the polymer, the hydrophobicity of the polymer decreased because the cationic groups provided hydrophilicity to the polymer. Therefore, polymer-modified beads with appropriate cationic and hydrophobic properties should be used to analyze each drug.
Temperature-responsive mixed-mode chromatography using mixed beads effectively exploits electrostatic interactions (Fig. 5) [114, 115]. Mixed-mode columns using PNIPAAm-modified beads and poly(3-acrylamidopropyltrimethylammonium chloride) (PAPTAC) modified beads have been investigated for temperature-modulated interactions with antiepileptic drugs, which often require TDM [114]. In the mixed mode column, PNIPAAm modified beads were used for temperature-modulated hydrophobic interaction with analytes. In addition, PAPTAC modified beads were used for electrostatic interaction with analytes because PAPTAC has strong cationic property [64, 77, 116]. Using a temperature-responsive mixed-mode column, the antiepileptic drugs phenytoin, clonazepam, nitrazepam, carbamazepine, lamotrigine, phenobarbital, zonisamide, and ethosuximide are retained in the column through electrostatic and hydrophobic interactions. The retention time of these antiepileptic drugs increased with increasing temperature because of the increased hydrophobic interactions with the drugs at higher temperatures in addition to the electrostatic interactions. Additionally, mixtures of zonisamide, carbamazepine, nitrazepam, and clonazepam were separated using a mixed-mode column (Fig. 5C). The results indicate that the prepared temperature-responsive mixed-mode column can be utilized for the TDM of multidrug doses of various antiepileptic drugs.
Temperature-responsive mixed-mode chromatography for TDM. (A) Conceptual diagram of the temperature-responsive mixed-mode column using thermoresponsive polymer-modified silica beads and cationic polymer-modified silica beads, (B) Temperature-modulated interactions between polymers and analytes, and (C) Chromatograms of antiepileptic drugs on the temperature-responsive mixed-mode column at various temperatures
Sample preparation using temperature-responsive spin column
In most cases of TDM using chromatography, sample preparation is required to remove serum proteins [117]. Various sample preparation methods, such as organic protein precipitation, solid extraction columns, and spin columns, require organic solvents that are not desirable for use in hospitals. Therefore, a sample preparation method that does not require organic solvents is desired. To resolve this issue, a temperature-responsive spin column without an organic solvent was developed for sample preparation [118] (Fig. 6). Two types of beads were prepared as the packing materials for the spin column (Fig. 6A). One was P(NIPAAm-co-DMAPAAm-co-BMA)-modified silica beads for trapping serum proteins. The beads were prepared using relatively large diameter of silica beads (40–63 μm). The other comprised P(NIPAAm-co-BMA)-modified silica beads for drug adsorption. The beads were prepared using relatively small diameter of silica beads (5 μm). The prepared two types of beads were packed into the spin columns as two layers; the prepared P(NIPAAm-co-BMA) beads and P(NIPAAm-co-DMAPAAm-co-BMA) beads were the bottom and upper layers of the spin column. Using the prepared spin column, the sample preparation of serum-voriconazole sample was performed (Fig. 6C). At 40 °C, the sample of voriconazole with serum protein was introduced to the spin column, and the spin column was centrifuged. The serum protein and voriconazole were adsorbed on the spin column at 40 °C. By reducing the temperature of the spin column and introducing cooling water into the column, the adsorbed drug was eluted from the column, whereas the protein adsorbed on the beads remained on the column. The efficacy of the spin column sample preparation was investigated by comparing protein precipitation using organic solvents (Fig. 6D). The voriconazole-serum protein samples were treated using a temperature-responsive spin column, followed by chromatographic analysis using an ODS column. Only a voriconazole peak was observed in the chromatogram after sample preparation using the spin column. By contrast, a contaminant peak was observed in the chromatogram after sample preparation via protein precipitation. The results indicate that the developed temperature-responsive spin column would be useful for sample preparation in healthcare facilities without organic solvents.
Temperature-responsive spin column for the preparation of drug-serum samples. (A) Temperature-responsive spin columns composed of two types of thermoresponsive beads, (B) Conceptual diagram of the temperature-responsive spin column, (C) Elution of serum proteins and voriconazole, and (D) Chromatograms of voriconazole and serum proteins prepared using protein precipitation and temperature-responsive spin column (adapted from [118] with permission from Elsevier)
Antibody drug separation using thermoresponsive polymer
Biopharmaceuticals have become effective medical treatments for intractable diseases [119]. Antibody drugs are promising biopharmaceutics because of their specificity for target molecules and relatively few side effects compared to low-molecular-weight chemical drugs [119,120,121]. The importance of separation and analysis techniques for antibody drugs has increased significantly. Numerous separation and analytical techniques have been developed for this purpose. Affinity chromatography, which employs a protein A ligand, is a powerful tool for this purpose because of the strong affinity between protein A and antibodies. Antibody elution from the columns is typically accomplished using low-pH aqueous solutions. The elution process in the antibody-drug separation column may result in the loss of activity of the antibody drugs and contamination owing to the elution of protein A. Therefore, there is a need for an antibody-drug separation column that employs distinct retention and elution mechanisms other than protein A.
Temperature-responsive chromatography using PNIPAAm is a potential technique for separating antibodies because of the ability to adjust analyte retention and elution by altering the temperature of the chromatographic system.
A temperature-responsive chromatography column for antibody-drug separation was developed using PNIPAAm containing a sulfonic acid group [66] (Fig. 7). Poly(NIPAAm-co-2-acrylamido-2-methylpropanesulfonic acid (AMPS)-co-BMA)-modified silica beads were prepared by atom transfer radical polymerization (ATRP) (Fig. 7A). Using the rituximab elution profiles, the prepared bead-packed column was investigated as an antibody purification column. Rituximab is adsorbed onto the copolymer brush at high temperatures through hydrophobic and electrostatic interactions because the copolymer dehydrates and becomes hydrophobic, and the anionic properties of the AMPS are maintained. By contrast, rituximab was not adsorbed onto the copolymer brush at low temperatures because it was extended and hydrated. The purification of rituximab from contaminants, albumin or hybridoma culture media, was achieved by simply changing the temperature of the column from 40 to 10 °C (Fig. 7B). Furthermore, three types of antibody-drug mixtures, rituximab, cetuximab, and bevacizumab, were separated using a column through temperature-modulated hydrophobic and electrostatic interactions (Fig. 7C). These findings indicate that a temperature-responsive column can be employed to separate and analyze biopharmaceuticals by simply controlling the column temperature.
Temperature-responsive chromatography using thermoresponsive anionic polymer-grafted silica beads for antibody drug purification. (A) Preparation scheme for thermoresponsive anionic polymer-grafted silica beads, (B) Temperature-modulated purification of rituximab from albumin contaminants, and (C) Separation of the three antibody drugs according to temperature using the prepared column
Another type of antibody–drug purification column were developed by using a mixed polymer brush composed of PNIPAAm and poly(4-vinyl pyridine)(P4VP) [68] (Fig. 8). P4VP has been utilized as a ligand for protein purification columns [122, 123]. Thus, mixed PNIPAAm and P4VP brush-modified silica beads were prepared as packing materials for effective antibody–drug purification (Fig. 8). A thermoresponsive mixed polymer brush was prepared by reversible addition-fragmentation chain transfer (RAFT) polymerization of 4VP and subsequent ATRP of NIPAAm (Fig. 8A). The elution behavior of rituximab was observed at various temperatures using a bead-packed column. At high temperatures, rituximab was adsorbed on the mixed polymer brush because PNIPAAm shrunk and P4VP was exposed, leading to an increased interaction between rituximab and P4VP (Fig. 8B). By contrast, at low temperatures, rituximab did not adsorb onto the column because PNIPAAm was extended, and P4VP was concealed, preventing the interaction between rituximab and P4VP. Rituximab was purified from the contaminant using a mixed polymer brush (Fig. 8B). A mixture of rituximab and albumin was introduced to the bead-packed column at 40 °C; rituximab was adsorbed on the mixed polymer brush, whereas albumin was not adsorbed and eluted from the column. The adsorbed rituximab was eluted from the column by reducing the temperature from 40 to 10 °C (Fig. 8B).
Temperature-responsive chromatography using a thermoresponsive mixed polymer brush for antibody drug purification. (A) Preparation scheme of thermoresponsive mixed-polymer brush-grafted silica beads and (B) Temperature-modulated purification of rituximab from albumin contaminants (adapted from [68] with permission from Elsevier)
Conclusions
This review summarizes recently developed temperature-responsive chromatography systems for clinical applications and their properties. The PNIPAAm-modified silica bead-packed column effectively separated drugs and serum proteins, allowing the determination of the quantity of drugs in the serum sample. In addition, quantitative analysis of drugs in serum samples was performed using a thermoresponsive cationic copolymer-modified bead-packed column. The antiepileptic drugs were separated using a temperature-responsive mixed-mode chromatography column. Serum sample preparation without organic solvent was performed using the developed temperature-responsive spin column. Antibody drugs were purified from contaminants using the developed temperature-responsive chromatography column by simply changing the temperature. Therefore, the developed PNIPAAm-based temperature-responsive chromatography system has the potential to function as an effective separation and analytical method for various types of medicines, including small-molecule drugs and biopharmaceuticals. These temperature-responsive chromatography systems can also be utilized for clinical diagnosis, as they can assess multiple medicines simultaneously. This highlights the significant potential of temperature-responsive chromatography in medicine and healthcare.
Data availability
The data used in the current study are available from the corresponding author upon reasonable request.
References
L. Ge, S.-P. Li, G. Lisak, Advanced sensing technologies of phenolic compounds for pharmaceutical and biomedical analysis. J. Pharm. Biomed. Analysis 179, 112913 (2020)
Y. Yang, Y. Chen, H. Tang, N. Zong, X. Jiang, Microfluidics for biomedical analysis. Small Methods 4, 1900451 (2020)
R. Koncki, Recent developments in potentiometric biosensors for biomedical analysis. Anal. Chim. Acta 599, 7–15 (2007)
N. Nuchtavorn, W. Suntornsuk, S.M. Lunte, L. Suntornsuk, Recent applications of microchip electrophoresis to biomedical analysis. J. Pharm. Biomed. Anal. 113, 72–96 (2015)
C. Citti, D. Braghiroli, M.A. Vandelli, G. Cannazza, Pharmaceutical and biomedical analysis of cannabinoids: a critical review. J. Pharm. Biomed. Anal. 147, 565–579 (2018)
A.S. Gross, Best practice in therapeutic drug monitoring. Br. J. Clin. Pharm. 46, 95–99 (1998)
H.C. Ates, J.A. Roberts, J. Lipman, A.E.G. Cass, G.A. Urban, C. Dincer, On-site therapeutic drug monitoring. Trend. Biotech. 38, 1262–1277 (2020)
S.H.Y. Wong, Advances in liquid chromatography and related methodologies for therapeutic drug monitoring. J. Pharm. Biomed. Anal. 7, 1011–1032 (1989)
H. Kanazawa, K. Yamamoto, Y. Matsushima, N. Takai, A. Kikuchi, Y. Sakurai, T. Okano, Temperature-responsive chromatography using poly(N-isopropylacrylamide)-modified silica. Anal. Chem. 68, 100–105 (1996)
H. Kanazawa, T. Sunamoto, E. Ayano, Y. Matsushima, A. Kikuchi, T. Okano, Temperature-responsive chromatography using poly(N-isopropylacrylamide) hydrogel-modified silica. Anal. Sci. 18, 45–48 (2002)
E. Ayano, H. Kanazawa, Temperature-responsive smart packing materials utilizing multi-functional polymers. Anal. Sci. 30, 167–173 (2014)
M. Kanezawa, E. Ayano, H. Kanazawa, Y. Akiyama, T. Okano, Study of a capillary LC system using temperature-responsive polymer-modified packing materials. Chromatography 27, 111–115 (2006)
K. Nagase, H. Kanazawa, Temperature-responsive chromatography for bioseparations: a review. Anal. Chim. Acta 1138, 191–212 (2020)
E. Ayano, Y. Suzuki, T. Nishio, Y. Nagata, H. Kanazawa, K. Nagase, T. Okano, Liquid chromatography-mass spectrometric analysis of dehydroepiandrosterone and related steroids utilizing a temperature-responsive stationary phase. Chromatography 35, 131–138 (2014)
M. Akimaru, K. Okubo, Y. Hiruta, H. Kanazawa, Temperature-responsive solid-phase extraction column for biological sample pretreatment. Anal. Sci. 31, 881–886 (2015)
T. Mikuma, T. Kuroki, M. Yoshikawa, R. Uchida, Y. Hiruta, H. Kanazawa, Analysis of psychoactive drugs by temperature-responsive chromatography. Chromatography 38, 115–121 (2017)
K. Nagase, J. Kobayashi, A. Kikuchi, Y. Akiyama, H. Kanazawa, T. Okano, Thermoresponsive polymer brush on monolithic-silica-rod for the high-speed separation of bioactive compounds. Langmuir 27, 10830–10839 (2011)
K. Nagase, J. Kobayashi, A. Kikuchi, Y. Akiyama, H. Kanazawa, T. Okano, Thermally modulated cationic copolymer brush on monolithic silica rods for high-speed separation of acidic biomolecules. ACS Appl. Mater. Interfaces 5, 1442–1452 (2013)
K. Nagase, J. Kobayashi, A. Kikuchi, Y. Akiyama, H. Kanazawa, T. Okano, Monolithic silica rods grafted with thermoresponsive anionic polymer brushes for high-speed separation of basic biomolecules and peptides. Biomacromol 15, 1204–1215 (2014)
K. Nagase, J. Kobayashi, A. Kikuchi, Y. Akiyama, H. Kanazawa, T. Okano, Thermoresponsive hydrophobic copolymer brushes modified porous monolithic silica for high-resolution bioseparation. RSC Adv. 5, 66155–66167 (2015)
Y. Maekawa, N. Okamoto, Y. Okada, K. Nagase, H. Kanazawa, Green analytical method for the simultaneous analysis of cytochrome P450 probe substrates by poly(N-isopropylacrylamide)-based temperature-responsive chromatography. Sci. Rep. 10, 8828 (2020)
K. Nagase, Y. Umemoto, H. Kanazawa, Effect of pore diameter on the elution behavior of analytes from thermoresponsive polymer grafted beads packed columns. Sci. Rep. 11, 9976 (2021)
K. Nagase, J. Kobayashi, A. Kikuchi, Y. Akiyama, H. Kanazawa, T. Okano, Interfacial property modulation of thermoresponsive polymer brush surfaces and their interaction with biomolecules. Langmuir 23, 9409–9415 (2007)
K. Nagase, J. Kobayashi, A. Kikuchi, Y. Akiyama, H. Kanazawa, T. Okano, Effects of graft densities and chain lengths on separation of bioactive compounds by nanolayered thermoresponsive polymer brush surfaces. Langmuir 24, 511–517 (2008)
K. Nagase, J. Kobayashi, A. Kikuchi, Y. Akiyama, H. Kanazawa, T. Okano, Preparation of thermoresponsive cationic copolymer brush surfaces and application of the surface to separation of biomolecules. Biomacromol 9, 1340–1347 (2008)
K. Nagase, J. Kobayashi, A. Kikuchi, Y. Akiyama, M. Annaka, H. Kanazawa, T. Okano, Influence of graft interface polarity on hydration/dehydration of grafted thermoresponsive polymer brushes and steroid separation using all-aqueous chromatography. Langmuir 24, 10981–10987 (2008)
K. Nagase, M. Kumazaki, H. Kanazawa, J. Kobayashi, A. Kikuchi, Y. Akiyama, M. Annaka, T. Okano, Thermoresponsive polymer brush surfaces with hydrophobic groups for all-aqueous chromatography. ACS Appl. Mater. Interfaces 2, 1247–1253 (2010)
K. Nagase, A. Mizutani Akimoto, J. Kobayashi, A. Kikuchi, Y. Akiyama, H. Kanazawa, T. Okano, Effect of reaction solvent on the preparation of thermo-responsive stationary phase through a surface initiated atom transfer radical polymerization. J. Chromatogr. AChromatogr. A 1218, 8617–8628 (2011)
K. Nagase, J. Kobayashi, A. Kikuchi, Y. Akiyama, H. Kanazawa, T. Okano, High stability of thermoresponsive polymer-brush-grafted silica beads as chromatography matrices. ACS Appl. Mater. Interfaces 4, 1998–2008 (2012)
M. Heskins, J.E. Guillet, Solution properties of poly(N-isopropylacrylamide). J. Macromol. Sci. A 2, 1441–1455 (1968)
H.G. Schild, Poly(N-isopropylacrylamide): experiment, theory and application. Prog. Polym. Sci. 17, 163–249 (1992)
A. Halperin, M. Kröger, F.M. Winnik, Poly(N-isopropylacrylamide) phase diagrams: fifty years of research. Angew. Chem. Int. Ed.. Chem. Int. Ed. 54, 15342–15367 (2015)
L. Tang, L. Wang, X. Yang, Y. Feng, Y. Li, W. Feng, Poly(N-isopropylacrylamide)-based smart hydrogels: design, properties and applications. Prog. Mater. Sci. 115, 100702 (2021)
K. Nagase, Thermoresponsive interfaces obtained using poly(N-isopropylacrylamide)-based copolymer for bioseparation and tissue engineering applications. Adv. Colloid Interface Sci. 295, 102487 (2021)
K. Nagase, T. Onuma, M. Yamato, N. Takeda, T. Okano, Enhanced wettability changes by synergistic effect of micro/nanoimprinted substrates and grafted thermoresponsive polymer brushes. Macromol. Rapid Commun.. Rapid Commun. 36, 1965–1970 (2015)
K. Nagase, J. Matsuda, A. Takeuchi, Y. Ikemoto, Hydration and dehydration behaviors of poly(N-isopropylacrylamide)-grafted silica beads. Surf. Interfaces 40, 103058 (2023)
S. Cammas, K. Suzuki, C. Sone, Y. Sakurai, K. Kataoka, T. Okano, Thermo-responsive polymer nanoparticles with a core-shell micelle structure as site-specific drug carriers. J. Control. Release 48, 157–164 (1997)
M. Nakayama, T. Okano, Multi-targeting cancer chemotherapy using temperature-responsive drug carrier systems. React. Funct. Polym. 71, 235–244 (2011)
J. Akimoto, M. Nakayama, T. Okano, Temperature-responsive polymeric micelles for optimizing drug targeting to solid tumors. J. Control. Release 193, 2–8 (2014)
M. Nakayama, J. Akimoto, T. Okano, Polymeric micelles with stimuli-triggering systems for advanced cancer drug targeting. J. Drug Target. 22, 584–599 (2014)
J. Wang, E. Ayano, Y. Maitani, H. Kanazawa, Enhanced cellular uptake and gene silencing activity of siRNA using temperature-responsive polymer-modified liposome. Int. J. Pharm. 523, 217–228 (2017)
K. Nagase, M. Hasegawa, E. Ayano, Y. Maitani, H. Kanazawa, Effect of polymer phase transition behavior on temperature-responsive polymer-modified liposomes for siRNA transfection. Int. J. Mol. Sci. 20, 430 (2019)
M. Maekawa-Matsuura, K. Fujieda, Y. Maekawa, T. Nishimura, K. Nagase, H. Kanazawa, LAT1-targeting thermoresponsive liposomes for effective cellular uptake by cancer cells. ACS Omega 4, 6443–6451 (2019)
R. Nemoto, K. Fujieda, Y. Hiruta, M. Hishida, E. Ayano, Y. Maitani, K. Nagase, H. Kanazawa, Liposomes with temperature-responsive reversible surface properties. Colloids Surf. B 176, 309–316 (2019)
T. Mori, M. Maeda, Temperature-responsive formation of colloidal nanoparticles from Poly(N-isopropylacrylamide) grafted with single-stranded DNA. Langmuir 20, 313–319 (2004)
D. Miyamoto, Z. Tang, T. Takarada, M. Maeda, Turbidimetric detection of ATP using polymeric micelles and DNA aptamers. Chem. Commun.Commun. 45, 4743–4745 (2007)
Z. Tang, T. Takarada, M. Maeda, Non-cross-linking aggregation of DNA-carrying polymer micelles triggered by duplex formation. Langmuir 34, 14899–14910 (2018)
N. Uehara, Y. Numanami, T. Oba, N. Onishi, X. Xie, Thermal-induced immuno-nephelometry using gold nanoparticles conjugated with a thermoresponsive polymer for the detection of avidin. Anal. Sci. 31, 495–501 (2015)
H. Kobayashi, M. Nishikawa, C. Sakamoto, T. Nishio, H. Kanazawa, T. Okano, Dual Temperature- and pH-responsive fluorescence molecular probe for cellular imaging utilizing a PNIPAAm-fluorescein copolymer. Anal. Sci. 25, 1043–1047 (2009)
Y. Hiruta, M. Shimamura, M. Matsuura, Y. Maekawa, T. Funatsu, Y. Suzuki, E. Ayano, T. Okano, H. Kanazawa, Temperature-responsive fluorescence polymer probes with accurate thermally controlled cellular uptakes. ACS Macro Lett. 3, 281–285 (2014)
M. Matsuura, M. Ohshima, Y. Hiruta, T. Nishimura, K. Nagase, H. Kanazawa, LAT1-targeting thermoresponsive fluorescent polymer probes for cancer cell imaging. Int. J. Mol. Sci. 19, 1646 (2018)
Y. Hiruta, Poly(N-isopropylacrylamide)-based temperature- and pH-responsive polymer materials for application in biomedical fields. Polym. J. 54, 1419–1430 (2022)
R. Yoshida, Creation of softmaterials based on self-oscillating polymer gels. Polym. J. 54, 827–849 (2022)
R. Tamate, A. Mizutani Akimoto, R. Yoshida, Recent advances in self-oscillating polymer material systems. Chem. Rec. 16, 1852–1867 (2016)
T. Masuda, A.M. Akimoto, K. Nagase, T. Okano, R. Yoshida, Design of self-oscillating polymer brushes and control of the dynamic behaviors. Chem. Mater. 27, 7395–7402 (2015)
K. Homma, T. Masuda, A.M. Akimoto, K. Nagase, K. Itoga, T. Okano, R. Yoshida, Fabrication of micropatterned self-oscillating polymer brush for direction control of chemical waves. Small 13, 1700041 (2017)
T. Masuda, A.M. Akimoto, M. Furusawa, R. Tamate, K. Nagase, T. Okano, R. Yoshida, Aspects of the Belousov–Zhabotinsky reaction inside a self-oscillating polymer brush. Langmuir 34, 1673–1680 (2018)
K. Homma, T. Masuda, A.M. Akimoto, K. Nagase, T. Okano, R. Yoshida, Stable and prolonged autonomous oscillation in a self-oscillating polymer brush prepared on a porous glass substrate. Langmuir 35, 9794–9801 (2019)
K. Homma, Y. Ohta, K. Minami, G. Yoshikawa, K. Nagase, A.M. Akimoto, R. Yoshida, Autonomous nanoscale chemomechanical oscillation on the self-oscillating polymer brush surface by precise control of graft density. Langmuir 37, 4380–4386 (2021)
N.S. Terefe, O. Glagovskaia, K. De Silva, R. Stockmann, Application of stimuli responsive polymers for sustainable ion exchange chromatography. Food Bioprod. Process. 92, 208–225 (2014)
K. Nagase, J. Kobayashi, A. Kikuchi, Y. Akiyama, H. Kanazawa, T. Okano, Thermally-modulated on/off-adsorption materials for pharmaceutical protein purification. Biomaterials 32, 619–627 (2011)
K. Nagase, S.F. Yuk, J. Kobayashi, A. Kikuchi, Y. Akiyama, H. Kanazawa, T. Okano, Thermo-responsive protein adsorbing materials for purifying pharmaceutical protein on exposed charging surface. J. Mater. Chem. 21, 2590–2593 (2011)
K. Nagase, J. Kobayashi, A. Kikuchi, Y. Akiyama, H. Kanazawa, T. Okano, Thermoresponsive anionic block copolymer brushes with a strongly anionic bottom segment for effective interactions with biomolecules. RSC Adv. 6, 93169–93179 (2016)
K. Nagase, J. Kobayashi, A. Kikuchi, Y. Akiyama, H. Kanazawa, T. Okano, Protein separations via thermally responsive ionic block copolymer brush layers. RSC Adv. 6, 26254–26263 (2016)
K. Okubo, K. Ikeda, A. Oaku, Y. Hiruta, K. Nagase, H. Kanazawa, Protein purification using solid-phase extraction on temperature-responsive hydrogel-modified silica beads. J. Chromatogr. A 1568, 38–48 (2018)
K. Nagase, S. Ishii, K. Ikeda, S. Yamada, D. Ichikawa, A. Akimoto, Y. Hattori, H. Kanazawa, Antibody drug separation using thermoresponsive anionic polymer brush modified beads with optimised electrostatic and hydrophobic interactions. Sci. Rep. 10, 11896 (2020)
K. Nagase, S. Kitazawa, T. Kogure, S. Yamada, K. Katayama, H. Kanazawa, Viral vector purification with thermoresponsive-anionic mixed polymer brush modified beads-packed column. Sep. Purif. Technol. 286, 120445 (2022)
K. Nagase, S. Ishii, A. Takeuchi, H. Kanazawa, Temperature-modulated antibody drug separation using thermoresponsive mixed polymer brush-modified stationary phase. Sep. Purif. Technol. 299, 121750 (2022)
K. Nagase, K. Yamazaki, Y. Maekawa, H. Kanazawa, Thermoresponsive bio-affinity interfaces for temperature-modulated selective capture and release of targeted exosomes. Mater. Today Bio 18, 100521 (2023)
A. Mizutani, K. Nagase, A. Kikuchi, H. Kanazawa, Y. Akiyama, J. Kobayashi, M. Annaka, T. Okano, Effective separation of peptides using highly dense thermo-responsive polymer brush-grafted porous polystyrene beads. J. Chromatogr. B 878, 2191–2198 (2010)
K. Nagase, J. Kobayashi, A. Kikuchi, Y. Akiyama, H. Kanazawa, T. Okano, Thermoresponsive anionic copolymer brushes containing strong acid moieties for effective separation of basic biomolecules and proteins. Biomacromol 15, 3846–3858 (2014)
K. Nagase, S. Kitazawa, S. Yamada, A.M. Akimoto, H. Kanazawa, Mixed polymer brush as a functional ligand of silica beads for temperature-modulated hydrophobic and electrostatic interactions. Anal. Chim. Acta 1095, 1–13 (2020)
D. Nomoto, K. Nagase, Y. Nakamura, H. Kanazawa, D. Citterio, Y. Hiruta, Anion species-triggered antibody separation system utilizing a thermo-responsive polymer column under optimized constant temperature. Colloids Surf. B 205, 111890 (2021)
Y. Maekawa, K. Yamazaki, M. Ihara, K. Nagase, H. Kanazawa, Simultaneous analysis of multiple oligonucleotides by temperature-responsive chromatography using a poly(N-isopropylacrylamide)-based stationary phase. Anal. Bioanal. Chem. 412, 5341–5351 (2020)
K. Nagase, A. Kimura, T. Shimizu, K. Matsuura, M. Yamato, N. Takeda, T. Okano, Dynamically cell separating thermo-functional biointerfaces with densely packed polymer brushes. J. Mater. Chem. 22, 19514–19522 (2012)
K. Nagase, Y. Hatakeyama, T. Shimizu, K. Matsuura, M. Yamato, N. Takeda, T. Okano, Hydrophobized thermoresponsive copolymer brushes for cell separation by multistep temperature change. Biomacromol 14, 3423–3433 (2013)
K. Nagase, Y. Hatakeyama, T. Shimizu, K. Matsuura, M. Yamato, N. Takeda, T. Okano, Thermoresponsive cationic copolymer brushes for mesenchymal stem cell separation. Biomacromol 16, 532–540 (2015)
K. Nagase, Y. Sakurada, S. Onizuka, T. Iwata, M. Yamato, N. Takeda, T. Okano, Thermoresponsive polymer-modified microfibers for cell separations. Acta Biomater. 53, 81–92 (2017)
K. Nagase, R. Shukuwa, T. Onuma, M. Yamato, N. Takeda, T. Okano, Micro/nano-imprinted substrates grafted with a thermoresponsive polymer for thermally modulated cell separation. J. Mater. Chem. B 5, 5924–5930 (2017)
K. Nagase, A. Ota, T. Hirotani, S. Yamada, A.M. Akimoto, H. Kanazawa, Thermoresponsive cationic block copolymer brushes for temperature-modulated stem cell separation. Macromol. Rapid Commun.. Rapid Commun. 41, 2000308 (2020)
T.-C. Sung, H.C. Su, Q.-D. Ling, S.S. Kumar, Y. Chang, S.-T. Hsu, A. Higuchi, Efficient differentiation of human pluripotent stem cells into cardiomyocytes on cell sorting thermoresponsive surface. Biomaterials 253, 120060 (2020)
K. Nagase, N. Uchikawa, T. Hirotani, A.M. Akimoto, H. Kanazawa, Thermoresponsive anionic copolymer brush-grafted surfaces for cell separation. Colloids Surf. B 185, 110565 (2020)
K. Nagase, N. Kojima, M. Goto, T. Akaike, H. Kanazawa, Thermoresponsive block copolymer brush for temperature-modulated hepatocyte separation. J. Mater. Chem. B 10, 8629–8641 (2022)
K. Nagase, H. Wakayama, J. Matsuda, N. Kojima, H. Kanazawa, Thermoresponsive mixed polymer brush to effectively control the adhesion and separation of stem cells by altering temperature. Mater. Today Bio 20, 100627 (2023)
K. Nagase, R. Shukuwa, H. Takahashi, N. Takeda, T. Okano, Enhanced mechanical properties and cell separation with thermal control of PIPAAm-brushed polymer-blend microfibers. J. Mater. Chem. B 8, 6017–6026 (2020)
K. Nagase, M. Shimura, R. Shimane, K. Hanaya, S. Yamada, A.M. Akimoto, T. Sugai, H. Kanazawa, Selective capture and non-invasive release of cells using a thermoresponsive polymer brush with affinity peptides. Biomater. Sci. 9, 663–674 (2021)
K. Nagase, N. Mukae, A. Kikuchi, T. Okano, Thermally modulated retention of lymphocytes on polymer-brush-grafted glass beads. Macromol. Biosci.. Biosci. 12, 333–340 (2012)
K. Nagase, D. Inanaga, D. Ichikawa, A. Mizutani Akimoto, Y. Hattori, H. Kanazawa, Temperature-modulated cell-separation column using temperature-responsive cationic copolymer hydrogel-modified silica beads. Colloids Surf. B 178, 253–262 (2019)
K. Nagase, G. Edatsune, Y. Nagata, J. Matsuda, D. Ichikawa, S. Yamada, Y. Hattori, H. Kanazawa, Thermally-modulated cell separation columns using a thermoresponsive block copolymer brush as a packing material for the purification of mesenchymal stem cells. Biomater. Sci. 9, 7054–7064 (2021)
K. Nagase, A. Okada, J. Matsuda, D. Ichikawa, Y. Hattori, H. Kanazawa, A thermoresponsive cationic block copolymer brush-grafted silica bead interface for temperature-modulated separation of adipose-derived stem cells. Colloids Surf. B 220, 112928 (2022)
N. Yamada, T. Okano, H. Sakai, F. Karikusa, Y. Sawasaki, Y. Sakurai, Thermo-responsive polymeric surfaces; control of attachment and detachment of cultured cells. Makromol. Chem. Rapid Commun.. Chem. Rapid Commun. 11, 571–576 (1990)
T. Okano, N. Yamada, M. Okuhara, H. Sakai, Y. Sakurai, Mechanism of cell detachment from temperature-modulated, hydrophilic-hydrophobic polymer surfaces. Biomaterials 16, 297–303 (1995)
M. Ebara, M. Yamato, M. Hirose, T. Aoyagi, A. Kikuchi, K. Sakai, T. Okano, Copolymerization of 2-carboxyisopropylacrylamide with N-isopropylacrylamide accelerates cell detachment from grafted surfaces by reducing temperature. Biomacromol 4, 344–349 (2003)
M. Ebara, M. Yamato, T. Aoyagi, A. Kikuchi, K. Sakai, T. Okano, Immobilization of cell-adhesive peptides to temperature-responsive surfaces facilitates both serum-free cell adhesion and noninvasive cell harvest. Tissue Eng. 10, 1125–1135 (2004)
Y. Akiyama, A. Kikuchi, M. Yamato, T. Okano, Ultrathin poly(N-isopropylacrylamide) grafted layer on polystyrene surfaces for cell adhesion/detachment control. Langmuir 20, 5506–5511 (2004)
A. Mizutani, A. Kikuchi, M. Yamato, H. Kanazawa, T. Okano, Preparation of thermoresponsive polymer brush surfaces and their interaction with cells. Biomaterials 29, 2073–2081 (2008)
K. Nagase, M. Watanabe, A. Kikuchi, M. Yamato, T. Okano, Thermo-responsive polymer brushes as intelligent biointerfaces: preparation via ATRP and characterization. Macromol. Biosci.. Biosci. 11, 400–409 (2011)
H. Takahashi, M. Nakayama, M. Yamato, T. Okano, Controlled chain length and graft density of thermoresponsive polymer brushes for optimizing cell sheet harvest. Biomacromol 11, 1991–1999 (2010)
Y. Arisaka, J. Kobayashi, M. Yamato, Y. Akiyama, T. Okano, Switching of cell growth/detachment on heparin-functionalized thermoresponsive surface for rapid cell sheet fabrication and manipulation. Biomaterials 34, 4214–4222 (2013)
Y. Arisaka, J. Kobayashi, K. Ohashi, K. Tatsumi, K. Kim, Y. Akiyama, M. Yamato, T. Okano, A heparin-modified thermoresponsive surface with heparin-binding epidermal growth factor-like growth factor for maintaining hepatic functions in vitro and harvesting hepatocyte sheets. Reg. Ther. 3, 97–106 (2016)
A.M. Akimoto, E. Hasuike, H. Tada, K. Nagase, T. Okano, H. Kanazawa, R. Yoshida, Design of tetra-arm PEG-crosslinked thermoresponsive hydrogel for 3D cell culture. Anal. Sci. 32, 1203–1205 (2016)
Y. Akiyama, Influence of poly(N-isopropylacrylamide) (PIPAAm) graft density on properties of PIPAAm grafted poly(dimethylsiloxane) surfaces and their stability. Heliyon 7, e06520 (2021)
V. Capella, R.E. Rivero, A.C. Liaudat, L.E. Ibarra, D.A. Roma, F. Alustiza, F. Mañas, C.A. Barbero, P. Bosch, C.R. Rivarola, N. Rodriguez, Cytotoxicity and bioadhesive properties of poly-N-isopropylacrylamide hydrogel. Heliyon 5, e01474 (2019)
A. Akimoto, E. Niitsu, K. Nagase, T. Okano, H. Kanazawa, R. Yoshida, Mesenchylmal stem cell culture on poly(N-isopropylacrylamide) hydrogel with repeated thermo-stimulation. Int. J. Mol. Sci. 19, 1253 (2018)
M. Nakao, K. Kim, K. Nagase, D.W. Grainger, H. Kanazawa, T. Okano, Phenotypic traits of mesenchymal stem cell sheets fabricated by temperature-responsive cell culture plate: structural characteristics of MSC sheets. Stem Cell Res. Ther. 10, 353 (2019)
M. Nakao, D. Inanaga, K. Nagase, H. Kanazawa, Characteristic differences of cell sheets composed of mesenchymal stem cells with different tissue origins. Reg. Ther. 11, 34–40 (2019)
M. Nakao, M. Matsui, K. Kim, N. Nishiyama, D.W. Grainger, T. Okano, H. Kanazawa, K. Nagase, Umbilical cord-derived mesenchymal stem cell sheets transplanted subcutaneously enhance cell retention and survival more than dissociated stem cell injections. Stem Cell Res. Ther. 14, 352 (2023)
M. Nakayama, Y. Toyoshima, A. Kikuchi, T. Okano, Micropatterned smart culture surfaces via multi-step physical coating of functional block copolymers for harvesting cell sheets with controlled sizes and shapes. Macromol. Biosci.. Biosci. 21, 2000330 (2021)
J. Kobayashi, T. Okano, Reproducible preparation of primary rat hepatocyte sheets using a thermoresponsive culture dish. Tissue Eng. C 29, 479–491 (2023)
K. Nagase, M. Nagaoka, Y. Nakano, R. Utoh, bFGF-releasing biodegradable nanoparticles for effectively engrafting transplanted hepatocyte sheet. J. Control. Release 366, 160–169 (2024)
K. Nagase, T. Nishiyama, M. Inoue, H. Kanazawa, Temperature responsive chromatography for therapeutic drug monitoring with an aqueous mobile phase. Sci. Rep. 11, 23508 (2021)
K. Nagase, S. Inoue, M. Inoue, H. Kanazawa, Two-dimensional temperature-responsive chromatography using a poly(N-isopropylacrylamide) brush-modified stationary phase for effective therapeutic drug monitoring. Sci. Rep. 12, 2653 (2022)
K. Nagase, H. Takagi, H. Nakada, H. Ishikawa, Y. Nagata, T. Aomori, H. Kanazawa, Chromatography columns packed with thermoresponsive-cationic-polymer-modified beads for therapeutic drug monitoring. Sci. Rep. 12, 12847 (2022)
K. Nagase, K. Matsumoto, H. Kanazawa, Temperature-responsive mixed-mode column for the modulation of multiple interactions. Sci. Rep. 12, 4434 (2022)
K. Nagase, M. Watanabe, F. Zen, H. Kanazawa, Temperature-responsive mixed-mode column containing temperature-responsive polymer-modified beads and anionic polymer-modified beads. Anal. Chim. Acta 1079, 220–229 (2019)
K. Nagase, M. Geven, S. Kimura, J. Kobayashi, A. Kikuchi, Y. Akiyama, D.W. Grijpma, H. Kanazawa, T. Okano, Thermoresponsive copolymer brushes possessing quaternary amine groups for strong anion-exchange chromatographic matrices. Biomacromol 15, 1031–1043 (2014)
R.D. McDowall, Sample preparation for biomedical analysis. J. Chromatogr. BChromatogr B 492, 3–58 (1989)
K. Nagase, Y. Ishizawa, M. Inoue, M. Kokubun, S. Yamada, H. Kanazawa, Temperature-responsive spin column for sample preparation using an all-aqueous eluent. Anal. Chim. Acta 1179, 338806 (2021)
G. Walsh, Biopharmaceutical benchmarks 2018. Nat. Biotechnol.Biotechnol. 36, 1136–1145 (2018)
P. Chames, M. Van Regenmortel, E. Weiss, D. Baty, Therapeutic antibodies: successes, limitations and hopes for the future. Br. J. Pharm. 157, 220–233 (2009)
J.G. Elvin, R.G. Couston, C.F. van der Walle, Therapeutic antibodies: market considerations, disease targets and bioprocessing. Int. J. Pharm. 440, 83–98 (2013)
E. Boschetti, Antibody separation by hydrophobic charge induction chromatography. Trend. Biotechnol. 20, 333–337 (2002)
Y. Li, Y. Sun, Poly(4-vinylpyridine): a polymeric ligand for mixed-mode protein chromatography. J. Chromatogr. A 1373, 97–105 (2014)
Acknowledgements
This study was supported in part by Grants-in-Aid for Scientific Research (grant numbers 19H02447, 20H05233, and 22H04560) from the Japan Society for the Promotion of Science (JSPS).
Funding
Open Access funding provided by Hiroshima University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no financial interests or personal relationships that could potentially affect the study described in this paper.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nagase, K. Bioanalytical technologies using temperature-responsive polymers. ANAL. SCI. 40, 827–841 (2024). https://doi.org/10.1007/s44211-024-00545-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44211-024-00545-3