Abstract
The present study uses maize flour and skimmed milk powder to develop a probiotic dairy-cereal-based food powder by spray drying and using response surface methodology for optimization. The processing parameters and ingredients, including inlet spray drying temperature (140–170 ℃), maize flour (80–120 g), and skim milk powder (60–80 g), were optimized against probiotic survivability count, moisture, sensory score, bulk density, and wettability as responses using response surface methodology. The optimum experimental conditions obtained to manufacture acceptable-quality powder were an inlet temperature of 151 ℃, an amount of maize flour of 102.74 g, and skim milk powder at 69.88 g. The probiotic survivability (8.35 log CFU/g) was observed at more than the recommended level (6.0 log CFU/g) due to the probiotic strain's microencapsulation by skim milk and maize flour components. The optimized powder had good nutritional and functional values and was observed to have acceptable water activity, surface structure, and color values. The shelf life of the product on the basis of probiotic survival (minimum 6.0 log CFU/g) was found to be 49 days at 4 ℃. Gastric and pancreatic survival was also observed at more than 50% in refrigerated conditions for up to 56 days of storage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Raabadi, or Rabadi, is a popular traditional fermented dairy-cereal grain-based beverage consumed daily in North-Western India, particularly in the rural parts of Haryana, Rajasthan, and Punjab [1, 2]. This nutritious staple food provides energy to millions of people with low to average incomes in India and contained approximately 0.65% fat, 8.7% total solids, and 2.2% protein [3]. One well-known variety of Raabadi is “Makka Ki Raab”, a dairy-cereal grain-based fermented product commonly prepared in southern Rajasthan, India, especially among the tribal communities. It is rich in nutrients, natural, and microbiologically safe, with appealing sensory attributes [2]. However, traditionally prepared Raabadi has a limited shelf life of one to two days at room temperature and may vary in sensory quality [1]. The shelf life of the product can be extended using drying techniques such as spray-drying and freeze-drying without destroying the beneficial microflora of Raabadi. This type of product can be used as a carrier of probiotic strains due to its constituents, such as milk and cereals, which are considered a good combination of encapsulating material as well as prebiotic [4]. The major ingredients of Raabadi are maize and buttermilk. Maize are a good source of starch, dietary fiber, and protein. It also contains potential prebiotic compounds such as resistant starch, β-glucans, arabinoxylans, phenolic substances, oligosaccharides (i.e., fructo and galacto), polypeptides, and other constituents [2, 5]. Starch is a versatile encapsulating material used to protect various compounds, including bacterial cells [6].
Buttermilk generally is by product of the butter industry. However, it can also be prepared using skim milk. Skim milk is rich in milk solids, including lactose and protein, along with ash, minerals, and trace elements. Its nutritional components and technological attributes, like emulsification and gel formation, make it an excellent microencapsulating material for probiotics. Previous studies have used skim milk as a carrier medium for probiotics, either alone or in combination with other wall materials [7], resulting in high probiotic cell survivability.
Currently, there is a significant focus on creating innovative dairy-cereal grain-based food products that incorporate probiotic microbes, known for their potential health attributes. The probiotic microflora exerts many health benefits, such as control and management of intestinal disorders, cholesterol reduction, anti-carcinogenic activity, and the potential to lower the risk of osteoporosis [8,9,10,11]. Lactobacillus helveticus MTCC 5463 is an Indian-origin indigenous strain with proven potential therapeutic application. This strain has been studied for its potential health benefits, including its ability to support digestive health, lower cholesterol [12], antioxidative properties [13], and boost the immune system [14]. Earlier, it has been used for the production of various probiotic foods such as chocolate [15], milk-finger millet fermented products [12], and symbiotic lassi with honey [16]. Considering the beneficial health effects, Lactobacillus helveticus was taken to develop the probiotic product in the study. Probiotic foods should contain sufficient amounts of viable cells to exert health benefits throughout processing and storage and must withstand the adverse environment of the human digestive system [17, 18]. One promising strategy to enhance probiotic viability is microencapsulation, which shields sensitive cells during food processing, ensuring their delivery in the gastrointestinal tract.
The food processing industry often requires preserving culture with long-term storage stability, which led to the development of microencapsulated food formulations and convenient ready-to-eat foods enriched with probiotics. Various techniques, such as freeze-drying, vacuum-drying, spray-drying, spray-chilling/cooling, fluidized bed coating, emulsion, and extrusion, have been used to microencapsulate the probiotic cells [4, 19]. The most widely used drying technique for preparing probiotic-dried foods is freeze-drying due to its low-temperature drying conditions. However, an alternative method, spray drying, is gaining attention due to its potential to reduce operational and manufacturing costs by up to 80%, enable continuous operation, and ensure yield stability. This process preserves several food characteristics, including nutritional composition, flavor, and color, mainly due to the short exposure time (5–100 s) to heat and the evaporative cooling effect. This technique is extensively studied for microencapsulation to enhance the stability of probiotic microbiota in different food matrices containing proteins, carbohydrates, polysaccharides, or their combinations [20]. The main drawback of spray drying is high-temperature processing, which can adversely impact the cell viability of probiotics. Nonetheless, numerous studies have explored the promise of spray-drying in developing probiotic foods and protecting microbial cells from adverse conditions during processing, storage, and transit through the gastrointestinal tract [21,22,23].
Although various researchers developed and characterized spray-dried probiotic food powders using LAB with probiotic attributes such as probiotic black carrot powder [24], guava juice powder [25], probiotic sohiong powder [26], none of these researchers developed the dairy-cereal grain-based fermented food beverage Raabadi locally known as "Makka ki Raab” as a dried food formulation after incorporation of beneficial LAB. Therefore, the current study aims to optimize the process development for the preparation of spray-dried probiotic dairy-cereal grain-based Raabadi beverage powder with long storage stability in terms of the survival of probiotics in gastric and pancreatic juice. Spray drying offers significant advantages for preparing probiotic food formulations. It enables the encapsulation of delicate probiotic bacteria, protecting them from moisture, oxygen, and light exposure, which can degrade their viability. The process ensures uniform encapsulation and particle size distribution, enhancing the stability and shelf life of the probiotics without altering their sensory properties. Consumption of optimized probiotic dairy-cereal grain-based powder will offer enhanced probiotic viability and delivery, diverse nutrient profiles, improved digestibility, and convenient consumption. This formulation will provide a stable source of probiotics with potential synergistic effects on gut health and overall well-being.
2 Material and methods
2.1 Proximate analysis
The pH, titratable acidity, moisture, ash, protein, crude fiber, crude fat, and total carbohydrate analysis were performed as per the standard protocol of the AOAC [27]. The total solid contents of skim milk powder (SMP) were determined using the difference method by Bunkar et al. [28]. The phenolic content was measured using Folin-Ciocalteu’s reagent method, as adopted by Bunkar et al. [29]. The total antioxidant activity was determined using as the method adopted by Bunkar et al. [29] using DPPH (2,2-Diphenyl-1-picrylhydrazyl) radical. The mineral content of Raabadi powder sample was analyzed by “atomic absorption spectrophotometry” (Shimadzu, Model AA-7000) using the protocol of Surve and Annapure [30].
2.2 Experimental plan
The changes in responses to probiotic count (colony forming unit/g), moisture (%), sensory score (hedonic scale: 0–9), bulk density (g/cm3), and wettability (s) were studied with three independent variables, viz. inlet temperature (0C), maize flour (g), and skim milk powder (g) using a central composite design (CCD). In this design, the actual experimental values were represented as coded values. For example, the inlet temperature ranges from 140 to 170 ℃, so the coded values for the inlet temperature were denoted as − 1 (lower value: 140 ℃), 0 (central value; 155 ℃), and + 1 (higher value: 170 ℃). Similarly, the coded values for maize flour and skim milk powder were expressed. There was a total of 19 experiments (trial runs) suggested, with 5 central point experiments in this design consisting of linear, square, and quadratic intercept terms. The best model was aimed at obtaining higher R2 and adjusted R2 values. The actual and predicted values show a better relationship when the model gives higher values of R2 and adjusted R2 [31].
A quadratic polynomial model was fitted to the experimental results, and the following equations were generated by regression analysis for Y1–5 as responses;
where Y1, Y2, Y3, Y4, and Y5 are the responses, probiotic count (log CFU/g), moisture (%), sensory score (Hedonic scale: 0–9), bulk density (g/cm3), and wettability (s), respectively;
β0 to β9 are the regression model coefficients.
A-coded values for spray drying inlet temperature (°C),
B-coded values for spray drying maize flour (g),
C-coded values for spray drying skim milk powder (g)
2.3 Product optimization
2.3.1 Preparation of culture and Raabadi curd
The ingredients, skim milk powder and maize (var. Pratap Sankar-3), were purchased from the local market. Maize grains were washed, cleaned, and spread on a stainless steel tray (40 × 65 cm) and dried in a tray dryer (Kheira Pvt. Ltd., India) to achieve a moisture level of around 10%, then ground in a low temperature to obtain maize flour. The probiotic culture Lactobacillus helveticus MTCC 5463 was used for Raabadi preparation. The strain was inoculated in MRS (de Mann Rogasa Sharpe) broth and incubated at 37 ℃ for 24 h to obtain a cell concentration of 6 log CFU/ml. The overnight grown culture in MRS broth was centrifuged at 8000 rpm for 10 min at 4 ℃. The cells were collected and washed with the phosphate saline buffer and used as the starter culture for manufacturing Raabadi. The biomass collected was introduced into maize flour and skim milk mixture at the inoculation stage and incubated at 37 ℃ for 48 h to achieve a probiotic viability count of 10 log CFU/ml [26]. The prepared Raabadi curd was thoroughly mixed with sterilized chilled water (1:1 v/v) for further processing.
2.3.2 Production of dairy-cereal grain-based probiotic Raabadi beverage powder (DCBPRP)
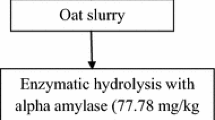
The viscous product was subjected to a colloid mill (ANJOPLUS Machines, Palghar, India) for proper mixing of flour particles for 10 min. After thorough mixing, the viscous product was strained through a muslin cloth for the removal of flour particles to avoid choking the atomizer of the spray drier. The liquid mixture was subjected to spray drying to obtain maize-based probiotic Raabadi powder. In this study, the pressure nozzle type spray dryer (ACEFIL Engineering Systems Pvt. Ltd., Vadodara, India) was used. The preliminary trials have been done to set the air pressure of the drying chamber, feed rate, feed temperature, aspirator speed, inlet, and outlet temperature to get a minimum 107 CFU/g probiotic count in the food powder. The powder was collected from a cyclone separator as prescribed by Vivek [32]. The cyclone separator was attached to the drying chamber, separating powder particles from the air powder mixture. The heavy particle-like powder was moving toward the bottom of the cyclone separator, and the air was moving to the upper side of the cyclone due to the density difference. The glass container was connected to the bottom of the cyclone separator to collect the powder. The optimized formulation was used for the production of bulk DCBPRP, and the samples were packed in 18 × 12 cm aluminum low-density polyethylene pouches having 2.7 ± 0.03 g weight and 69 ± 0.06 µm thickness using a semi-automatic heat sealing machine. The detailed flow diagram for Raabadi manufacturing is depicted in Fig. 1.
2.4 Response measurements
2.4.1 Probiotic survivability count
The probiotic count was determined using MRS agar after suitable serial dilution in a phosphate buffer solution in the powder during optimization trials. The count of powder during storage was analyzed at three different temperatures, i.e., 4, 25, and 37 ℃ in 7 days’ interval using the dilution method [33].
2.4.2 Bulk density, sensory evaluation, and wettability
The loose bulk density of the probiotic Raabadi powder was determined using the method adopted by Vivek [32] using the following formulae;
where, W1—weight of probiotic powder after tapping and W2—volume of bulk probiotic powder.
The sensory evaluation of reconstituted Raabadi powder samples was done using a 9-point hedonic scale ranging from 1 (“dislike extremely”) to 9 (“like extremely”) and was performed by maintaining the product temperature at 28 ± 2 ℃ and 70% relative humidity [29]. The wettability time of the probiotic powder sample was calculated using the procedure adopted by Arepally et al. [34]. 1 g of powder sample was evenly dispersed over the surface of 100 ml of distilled water at ambient temperature (25 ℃) and left undisturbed. The duration of time taken for the particles of the product to settle, submerge, and vanish was then recorded.
2.5 Optimization and validation
The different levels of ingredients were tried to obtain the most accepted probiotic Raabadi powder (DCBPRP). The product was optimized based on the level of skim milk powder, maize flour, and inlet temperature, and the responses (probiotic count, moisture, sensory score, bulk density, and wettability). The different combinations were evaluated using design expert software in order to obtain optimized spray drying conditions [31]. Further, the model's lack of fit values was also determined to assess the adequacy of the fitted model.
The best and most optimized sample was selected on the basis of the following criteria;
-
1.
Probiotic strain counts more than 107 log CFU/g
-
2.
Moisture content less than 5%
-
3.
Sensory scores more than 8 on the 9 point-hedonic scale
-
4.
Bulk density—maximum
-
5.
Wettability time—minimum
2.6 Powder characterization
2.6.1 Water activity
The water activity of the Raabadi powder samples was measured at 25 ℃ using the “LabTouch-aw” water activity meter (novasina, Lachen, Switzerland) according to the method suggested by Seth et al. [35]. The product temperature was maintained at 25 ℃, and about 3 g of sample was taken, which was enough to cover the filling indicator of the sample cup. The sample cup was placed on the sensor probe of the water activity meter properly, and readings were recorded.
2.6.2 Color
The color of the DCBPRP sample was analyzed using the Chroma Meter CR- 400 (Konica Minolta Optics, Japan) [36]. The results were expressed in L*, a*, and b* values, where L* represents light to dark, a* denotes red to green, and b* represents yellow to blue. The color intensity expressed in Chroma was determined by the formula given below;
The hue angle (H°) of the product sample was determined using the following formula;
2.6.3 Sample morphology
The powder sample was examined for morphology, surface, and microstructure using “field emission scanning electron microscopy” (FESEM) (JSM-7610FPlus, JEOL Ltd., USA) and operated at a voltage of 5 kV. The images were taken at magnification of 400, 600, 8000, and 15,000 ×.
2.7 Probiotic survival under gastric and pancreatic conditions during storage
In-vitro simulated gastric and pancreatic juices survival of probiotic strain of powder was assessed as per the method adopted by Meena et al. [37], with some minor modifications. The DCBPRP powder sample packed in pouches was stored at two storage conditions (i.e., 4 and 37 ℃) to check the survival of probiotics under simulated gastric and pancreatic juice conditions in the interval of 14 days. 1 g powder sample was taken in a 10 ml MRS broth test tube and the probiotic strain was allowed to grow overnight (24 h). Overnight-grown probiotic cells were centrifuged (8000g, 4 ℃, 10 min), and the cell pellets were washed twice. Then, the cell suspension was mixed in a solution mimicking gastric juice. The number of cells was adjusted to a specific level. The cell-gastric juice mixture was shaken for 3 h at body temperature (37 ℃) to simulate movement in the stomach. Samples were taken before and after shaking and diluted before being plated on a growth medium to enumerate the survival count.
The percentage of surviving organisms after exposure to simulated gastric juice was calculated using the formula given below:
where loggjuice0 and loggjuice3 have survival counts of 0 and 3 h incubation, respectively.
After spending 3 h in the simulated gastric juice, the cells were collected again by spinning them down in a centrifuge. They were washed with a phosphate buffer solution (PBS) to remove any leftover gastric juice. An equal amount of a solution mimicking pancreatic juice (PJ) was then added to the cells. This cell-pancreatic juice mixture was shaken continuously for 24 h at body temperature (37 ℃) to simulate movement in the gut. Samples were taken for survival count after adding the pancreatic juice (0 h) immediately and after 24 h of shaking. These samples were diluted and plated on a growth medium to enumerate the survival count. The survival percentage of culture samples was determined using the following formula;
where logPpanc.juice0 and logPpanc.juice24 represents survival count at 0 and 24 h incubation, respectively.
2.8 Statistical analysis
The experimental trials were conducted in triplicate (n = 3), and the values are tabulated as mean ± standard deviation. The statistical significance (p < 0.05) was calculated by one-way ANOVA (analysis of variance) using Duncan’s post hoc test using statistics analytical tool (SPSS version 16.0). The ANOVA test was employed for validation of the optimized formulation generated by the Response Surface Methodology to calculate significant differences (p < 0.05) among samples using the design expert software version 22.0. The graphs were plotted using OriginPro 2018 software.
3 Results and discussion
3.1 Proximate analysis of maize flour and skim milk powder
The maize flour used in the present study contained 11.76 ± 0.52, 3.29 ± 0.1, 9.05 ± 0.23, 1.14 ± 0.18, 73.45 ± 0.77, 1.17 ± 0.08 of percentage moisture, crude fat, crude protein, crude fiber, carbohydrates, and ash, respectively. The total phenol content (µmol gallic acid equivalents/g) and antioxidant properties (%) were found to be 21.37 ± 1.07 and 77.27 ± 1.41, respectively. The SMP used in the study had 2.93 ± 0.15, 0.93 ± 0.06, 36.38 ± 0.88, 7.28 ± 0.1, 52.47 ± 0.95, 97.07 ± 0.15 percentage moisture, fat, protein, ash, carbohydrate, and total solids, respectively. The SMP had a pH of 6.73 ± 0.06 and an acidity of 0.85 ± 0.05% in terms of % lactic acid. The SMP was free from any observable dust, smell, or any other abnormality.
3.2 Effect of process parameters on responses
The effect of independent variables and observed experimental values of responses are shown in Table 1. The interactive effect of an independent variable on the responses is distinguished using the means; therefore, it is expected to obtain higher F-values to nullify the null hypothesis [31]. The viability of probiotics (log CFU/g) was obtained in the range of 7.53 to 8.38. The probiotic viability count was significantly affected by inlet temperature, whereas a non-significant effect was observed for maize flour and skim milk powder in linear terms. A further significant effect was observed on probiotic viability for all three independent variables in square terms, whereas a non-significant effect of interactive terms was found. At varying temperatures, the intricate interplay between moisture content, heat transfer dynamics, and the biological characteristics of probiotic strains dictates their survival and viability. High inlet temperatures can trigger thermal stress, leading to protein denaturation and cell membrane damage, thereby compromising probiotic integrity [38]. Maize flour, abundant in carbohydrates and fibers, forms a barrier that shields probiotic cells from heat and mechanical stresses. SMP, rich in proteins and lactose, further reinforces this protection, reducing moisture loss and preventing cellular damage [6]. Together, these two ingredients might have acted as wall encapsulants for probiotics, which helped to retain higher viable cells in the Raabadi powder. The result of the current study is in agreement with the observations of other studies carried out on probiotic buttermilk powder [31], lassi powder [39], and sweetened yoghurt powder [35].
The moisture content in the experimental trials was observed in the range of 2.88 to 3.85%. In this study, the effect of inlet temperature and SMP on moisture content was significant in linear terms. There was a significant effect of interactive terms on inlet temperature and moisture content; however, only inlet temperature was significantly affected in squared terms. Higher inlet temperatures typically lead to faster evaporation rates, resulting in lower moisture content in the final product. Conversely, lower inlet temperatures may prolong drying times, potentially leading to higher moisture content [40]. Maize flour, rich in carbohydrates and fibers, aids in absorbing excess moisture, while SMP, with its proteins and lactose, contributes to moisture retention. Together, maize flour and SMP form a protective matrix around the probiotic cells, regulating moisture levels and reducing water activity within the product. A similar relationship between inlet spray drying temperature and moisture content was reported in probiotic buttermilk powder [31], lassi powder [39], and infant milk formula powder [40].
The sensory score was observed in the range of 7.54 to 8.24 during the evaluation of experimental products. The product was diluted 10 times before sensory evaluation. The sensory score is affected by inlet temperature, maize flour, and skim milk powder. In linear terms, sensory score significantly decreased with an increase in inlet spray drying temperature, whereas a significant positive relationship was found with maize flour. The effect of inlet temperature (− 0.23), maize flour (− 0.12), and skim milk powder (− 0.11) was observed with an inverse relationship with the sensory score in squared terms. Higher inlet temperatures can lead to browning reactions and flavor degradation, potentially compromising sensory attributes [40]. Maize flour and SMP contribute to the texture, mouthfeel, and flavor of the final product. However, excessive amounts of these ingredients might impart undesirable tastes or textures, adversely affecting the sensory characteristics of DCBPRP. The similar type of effect observed in sensory attributes of spray-dried lassi powder [39].
The wettability time (s) was increased with increasing the inlet temperature, maize flour, and skim milk powder. There was a significant (p < 0.05) increase in wettability with respect to inlet temperature and maize flour in linear terms, whereas all three independent variables had a significant impact in square terms. However, there was a non-significant relationship observed on wettability time for all three interaction terms. The wettability of powdered samples during experiments was observed in the range of 255 to 485 s. The wettability of soymilk powder prepared by spray drying had a large variation in the range of 22 to 1100 s [41], whereas blackberry powder varied from 82 to 134 s [42]. According to Nguyen et al. [41], the wettability of increases with an increase in inlet air temperature. The increased inlet temperature causes a decrease in the agglomeration ability of powder particles in the drying chamber. Therefore, small-sized particles are formed in the final powdered samples. The smaller particles have a large surface-to-mass ratio, which may not allow individual particles to wet easily; however, there is the chance to form clumps of the powder, resulting in a wetted surface layer. The wetted surface layer decreases the water penetration rate into the powder clumps. Thus, it increases the time to wet or absorb the water particles, resulting in a longer wettability time.
The bulk density in 19 different experimental trials was found to be in the range of 0.256 to 0.384 g/cm3. The bulk density decreased with an increase in inlet temperature, maize flour, and skim milk powder. The bulk density was significantly (p < 0.05) affected by inlet temperature (− 0.019), maize flour (+ 0.006), and skim milk powder (+ 0.0055) in linear terms as well as in squared terms. It may be attributed to the increased quantity of hollow particles and reduced moisture content in Raabadi powder samples. Higher inlet temperatures generally result in lower bulk densities due to increased moisture evaporation and particle shrinkage. Maize flour, with its fibrous structure and SMP, rich in proteins and carbohydrates, contributes to the formation of a cohesive matrix, potentially increasing bulk density. However, excessive incorporation of these ingredients can lead to higher bulk densities and reduced flowability. Wang et al. [43] reported that an increase in spray drying inlet temperature decreases the bulk density of soy sauce powder due to the increased tendency of powder particles to be hollow. It might be due to a greater evaporation rate or a lowered moisture content in the finished powdered product.
The model coefficients for the effect of spray drying conditions (inlet temperatures, maize flour, and skim milk powder) on responses are shown in Table 2. The effect of processing parameters and ingredients on the different responses is defined as the following quadratic polynomial equation:
The effect of interaction between independent variables on probiotic count, moisture, sensory score, bulk density, and wettability is plotted on 3D response surface plots and shown in Fig. 2.
3.3 Optimization and validation
The optimum experimental processing temperature and formulation ingredients were obtained using numerical optimization after the model fitting. The relative importance of individual independent variables and responses was assigned from 1 + (least desirable) to 5 + (most desirable). The relative importance of the probiotic viability count was assigned a maximum (5 +). Further, the model was validated by conducting experiments in triplicate under the optimized conditions, and the experimental results showed an error from 0.31 to 15.43%. The optimum experimental conditions obtained for DCBPRP were: inlet temperature at 151.37 ℃ (⁓ 151 ℃), amount of maize flour at 102.74 g, and skim milk powder at 69.88 g. The optimized predicted values for responses are given in Table 3. The overall desirability of the fitted model was 85.88%. The powdered sample was reconstituted in different water ratios to obtain the best product on the basis of sensory evaluation.
The optimized predicted values for responses, i.e., probiotic count (8.35 log CFU/g), moisture content (3.08%), sensory score (8.16), bulk density (0.38 g/cm3), and wettability (330.56 s) were observed. Rawat et al. [39] prepared a lassi powder at an inlet temperature of 190 ℃, an outlet temperature of 70 ℃, and a speed of 300 rpm with maximum survival of lactic acid bacteria count. Seth et al. [44] developed a sweetened yogurt at an inlet temperature of 148 ℃, a feed rate, 0.54 L/h, and an atomizer pressure of 898 kPa with good survival of bacterial cultures. Further, the model was validated by conducting experiments in triplicate under the optimized conditions, and the experimental results showed an error from 0.31 to 15.43% (Table 3).
3.4 Proximate characterization of DCBPRP
The optimized food powder (DCBPRP) contained moisture 3.28 ± 0.11%, fat 1.54 ± 0.22%, protein 29.32 ± 2.37%, ash 4.39 ± 0.08%, carbohydrate 59.8 ± 2.39%, and total solids 96.72 ± 0.11%. The total phenol content (µmol GAE)/g) and antioxidant properties (%) were found to be 36.04 ± 1.56 and 81.72 ± 1.1, respectively. The phosphorus, potassium, calcium, sodium, and magnesium content in the DCBPRP was observed at 1090.33 ± 6.03, 470.33 ± 6.03, 950.33 ± 15.01, 201.33 ± 6.03, and 120.33 ± 5.51 mg/100 g, respectively. The Raabadi powder contains a good amount of minerals in the optimized product. The organic matter content in the powdered sample at 550 ℃ was observed at 90.87 ± 2.57%. The optimized DCBPRP has a water activity of 0.268 ± 0.01. The food powders having initial water activity below 0.3 are considered good and safe during storage [22]. The color values L* (light to dark), a* red to green, and b* (yellow to blue) of DCBPRP were observed to be 92.03 ± 0.8, 1.29 ± 0.12, and 12.69 ± 0.41, respectively. The color intensity (Chroma) of the sample was found to be 12.76, and the Hue angle was 84.19.
3.5 Particle morphology of spray-dried DCBPRP
The images generated by FESEM of optimized Raabadi powder, prepared at optimized spray drying conditions, are shown in Fig. 3a–d with different magnification levels. Figure 3a, b shows the amorphous structure of powder particles with a spherical shape and smooth surface. However, upon closer inspection, it is noted that the particles have dents and small vacuoles in their interior, suggesting that the particles are not entirely homogeneous. Figure 3c, d shows encapsulated cells within the powder particles at 8000 and 15,000 × magnifications, respectively. No visible free probiotic cells are found in these images, which supports the claim that the encapsulation of cells occurred adequately during spray drying. Proper microencapsulation is a prerequisite for the probiotic strain to survive in spray-drying conditions [32]. Vivek et al. [26] reported a spray-dried sohiong powder with a spherical shape and small size particles. Earlier studies also reported that skim milk powders produced by spray drying had smooth surface structures with dents, while whole milk powders were observed with a similar type of surface morphology accompanied by bright surfaces [45]. During storage, the smoothness and spherical shape of the particles are reduced with the elongation of storage time. It may be attributed to the agglomeration of particles due to the formation of liquid bridges and an increase in moisture content [45].
3.6 Probiotic survival under gastric and pancreatic juice conditions during storage
The probiotic survival under gastric and pancreatic conditions at two different storage temperatures are shown in Table 4. The highest gastric and pancreatic survival was found at 0 days, which decreased significantly (p < 0.05) in the interval of 14 days for both storage conditions. The decreasing trend in survival was significantly (p < 0.05) higher at 37 ℃ than at 4 ℃ storage temperature. It might be attributed to lower survival of probiotic strain at higher temperature than refrigerated storage conditions. Pancreatic enzymes are released into the small intestine through the pancreatic duct, where they assist in breaking down proteins, carbohydrates, and lipids in the diet. The probiotic bacteria should withstand the presence of pancreatic enzymes. The results of study shown that probiotic strain used in powder preparation was able to survive in both stomach and pancreatic digestion, which is indicative of their ability to colonize the intestine. The survival in gastric and pancreatic digestion conditions was observed more than 50% and 27%, respectively, at 4 ℃ up to 56 days’ storage. Earlier, many previous studies claimed that probiotic strains of food powders were less resistant to pancreatic juice than gastric juice and showed better survivability at refrigerated storage temperatures [32, 37, 46].
The probiotic product must have a minimum number of 106 CFU/g or ml until the expiration of the declared shelf life, or the daily intake dose should be about 108 CFU/g [47]. The probiotic viability count was found to be 7.81 ± 0.03 CFU/g at 0 days. The survivability of probiotic strain in powder samples showed a decreasing trend during storage at different temperatures (data given in supplementary Table 1). The counts of probiotic strain at 4, 25, and 37 ℃ decreased significantly (p < 0.05) during storage in the interval of 7 days. The probiotic storage acceptability (˃106 CFU/g) was found to be highest when stored at 4 ℃. The probiotic counts in powder decreased up to 6.0 log CFU/g in 49 days at a storage temperature of 4 ℃, whereas the acceptable probiotic count at 25 and 37 ℃ were observed up to 35 and 28 days, respectively. Vivek et al. [32] reported the storage stability of probiotic count (Lactobacillus plantarum subsp. plantarum) in a spay-dried Sohiong powder for up to 36 days at room temperature, whereas 104 days were observed under refrigerated conditions. Ahlawat et al. [31] observed the count of probiotic Bifidobacterium bifidum more than 6 log CFU/g up to 4 weeks storage in spray-dried probiotic buttermilk powder at 4 ± 1 ℃ whereas one week at 27 ± 1 ℃.
4 Conclusion
Dairy-cereal grain-based food products are gaining tremendous consumer demand due to their diverse health-beneficial attributes. A dairy-cereal grain-based probiotic Raabadi beverage was developed using probiotic Lactobacillus helveticus MTCC 5463 and spray-dried to produce the probiotic powder. The optimization of ingredients and spray-drying conditions was achieved against responses such as probiotic viability, moisture content, bulk density, sensory score, and wettability through a central composite rotable design approach. The responses found significantly (p < 0.05) affected by the independent variables. The optimized beverage powder was found to be nutritionally protein-rich and contained a good amount of phenolics, antioxidants, and minerals. It had a probiotic viability count of more than 6 log10 CFU/g and acceptable physico-chemical properties. The product showed probiotic survivability for up to 49 days during storage at 4 ℃. Furthermore, this method has the potential to be adopted and scaled up for the commercial production of a dairy-cereal grain-based spray-dried probiotic Raabadi powder. The developed food powder may serve as a dairy-cereal grain-based ready-to-serve formulation that combines the nutritional richness of dairy and cereal grains with the probiotics, offering a convenient and tasty way to support digestive health. However, the efficacy of spray-dried probiotic Raabadi powder needs to be evaluated in an animal model before the commercialization of technology in the food processing industry.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its supplementary information files. Any raw data files be needed in another format they are available from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Sreeja V, Prajapati JB. Ethnic fermented foods and beverages of Gujarat and Rajasthan. In: Tamang JP, editor. ethnic fermented foods and beverages of India: science history and culture. Berlin: Springer; 2020. p. 157–87. https://doi.org/10.1007/978-981-15-1486-9_7.
Meena KK, Taneja NK, Jain D, Ojha A. Bioactive components and health benefits of maize-based fermented foods: a review. Biointerface Res Appl Chem. 2023;13(4):1–22. https://doi.org/10.33263/BRIAC134.338.
Modha H, Pal D. Optimization of Rabadi-like fermented milk beverage using pearl millet. J Food Sci Technol. 2011;48(2):190–6. https://doi.org/10.1007/s13197-010-0146-6.
Meena KK, Taneja NK, Ojha A, Meena S. Application of spray-drying and freeze-drying for microencapsulation of lactic acid bacteria: a review. Ann Phytomed. 2023;12(1):706–16. https://doi.org/10.54085/ap.2023.12.1.76.
Tomasik P, Tomasik P. Probiotics, non-dairy prebiotics and postbiotics in nutrition. Appl Sci. 2020;10(4):1–26. https://doi.org/10.3390/app10041470.
Hoyos-Leyva JD, Bello-Pérez LA, Alvarez-Ramirez J, Garcia HS. Microencapsulation using starch as wall material: a review. Food Rev Int. 2018;34(2):148–61. https://doi.org/10.1080/87559129.2016.1261298.
Kamil RZ, Yanti R, Murdiati A, Juffrie M, Rahayu ES. Microencapsulation of indigenous probiotic Lactobacillus plantarum Dad-13 by spray and freeze-drying: strain-dependent effect and its antibacterial property. Food Res. 2020;4(6):2181–9. https://doi.org/10.26656/fr.2017.4(6).280.
Chaudhari A, Dwivedi MK. The concept of probiotics, prebiotics, postbiotics, synbiotics, nutribiotics, and pharmabiotics. In: Dwivedi MK, Amaresan N, Sankaranarayanan A, Kemp EH, editors. probiotics in the prevention and management of human diseases. Cambridge: Academic Press; 2022. p. 1–11. https://doi.org/10.1016/b978-0-12-823733-5.00013-1.
Meena KK, Taneja NK, Jain D, Ojha A. Spontaneously fermented cereal based products: an ancient health promoting formulae for consumption of probiotic lactic acid bacteria. Biointerface Res Appl Chem. 2023;13(5):465–91. https://doi.org/10.33263/BRIAC135.465.
Shahein MR, Elkot WF, Albezrah NKA, Abdel-Hafez LJM, Alharbi MA, Massoud D, Elmahallawy EK. Insights into the microbiological and physicochemical properties of bio-frozen yoghurt made with probiotic strains in combination with Jerusalem Artichoke Tubers powder. Fermentation. 2022;8(8):390. https://doi.org/10.3390/fermentation8080390.
Alshafei MM, Mabrouk AM, Hanafi EM, Ramadan MM, Korany RMS, Kassem SS, Mohammed DM. Prophylactic supplementation of microencapsulated Boswellia serrata and probiotic bacteria in metabolic syndrome rats. Food Biosci. 2023;51(2):102325. https://doi.org/10.1016/j.fbio.2022.102325.
Chaudhary JK, Mudgal S. Antidiabetic and hypolipidemic action of finger millet (Eleusine coracana) enriched probiotic fermented milk: an in vivo rat study. Food Technol Biotechnol. 2020;58(2):192–202. https://doi.org/10.17113/FTB.58.02.20.6308.
Panchal G, Sakure A, Hati S. Peptidomic profiling of fermented goat milk: considering the fermentation-time dependent proteolysis by lactobacillus and characterization of novel peptides with antioxidative activity. J Food Sci Technol. 2022;59(6):2295–305. https://doi.org/10.1007/s13197-021-05243-w.
Senan S, Prajapati JB, Joshi CG. Feasibility of genome-wide screening for biosafety assessment of probiotics: a case study of Lactobacillus helveticus MTCC 5463. Probiotics Antimicrob Prot. 2015;7(4):249–58. https://doi.org/10.1007/s12602-015-9199-1.
Gadhiya D, Shah NP, Patel AR, Prajapati JB. Preparation and shelf life study of probiotic chocolate manufactured using Lactobacillus helveticus MTCC 5463. Acta Aliment. 2018;47(3):350–8. https://doi.org/10.1556/066.2018.47.3.11.
Shah C, Mokashe N, Mishra V. Preparation, characterization and in vitro antioxidative potential of synbiotic fermented dairy products. J Food Sci Technol. 2016;53(4):1984–92. https://doi.org/10.1007/s13197-016-2190-3.
Elkot WF. Preparation and properties of bio-yoghurt using Jerusalem Artichoke Tubers powder and different probiotic strains. Egypt J Dairy Sci. 2017;45(1):55–66.
Elkot WF, Elmahdy E, El-Sawah T, Alghamdia OA, Alhag SK, AL-Shahari EA, Al-Farga A, Ismail HA. Development and characterization of a novel flavored functional fermented whey-based sports beverage fortified with Spirulina platensis. Int J Biol Macromol. 2024;258(2):128999.
El Sayed HS, Mabrouk AM. Encapsulation of probiotics using mixed sodium alginate and rice flour to enhance their survivability in simulated gastric conditions and in UF-Kariesh cheese. Biocatal Agric Biotechnol. 2023;50(3):102738. https://doi.org/10.1016/j.bcab.2023.102738.
Liu H, Cui SW, Chen M, Li Y, Liang R, Xu F, Zhong F. Protective approaches and mechanisms of microencapsulation to the survival of probiotic bacteria during processing, storage and gastrointestinal digestion: a review. Crit Rev Food Sci Nutr. 2019;59(17):2863–78. https://doi.org/10.1080/10408398.2017.1377684.
Obradović N, Volić M, Nedović V, Rakin M, Bugarski B. Microencapsulation of probiotic starter culture in protein–carbohydrate carriers using spray and freeze-drying processes: implementation in whey-based beverages. J Food Eng. 2022. https://doi.org/10.1016/j.jfoodeng.2022.110948.
Nishad J, Selvan CJ, Mir SA, Bosco SJD. Effect of spray drying on physical properties of sugarcane juice powder (Saccharum officinarum L.). J Food Sci Technol. 2017;54(3):687–97. https://doi.org/10.1007/s13197-017-2507-x.
Agudelo-Chaparro J, Ciro-Velásquez HJ, Sepúlveda-Valencia JU, Pérez-Monterroza EJ. Microencapsulation of Lactobacillus rhamnosus ATCC 7469 by spray drying using maltodextrin, whey protein concentrate and trehalose. Food Sci Technol Int. 2021. https://doi.org/10.1177/10820132211020621.
Murali S, Patel AS, Kar A. Storage stability of encapsulated black carrot powder prepared using spray and freeze-drying techniques. Curr Agric Res J. 2019;7(2):261–7. https://doi.org/10.12944/carj.7.2.14.
Upadhyay R, Dass FP. Physicochemical analysis, microbial survivability, and shelf life study of spray-dried synbiotic guava juice powder. J Food Process Preserv. 2021;45(2):1–11. https://doi.org/10.1111/jfpp.15103.
Vivek K, Mishra S, Pradhan RC. Optimization of spray drying conditions for developing nondairy based probiotic sohiong fruit powder. Int J Fruit Sci. 2021;21(1):193–204. https://doi.org/10.1080/15538362.2020.1864567.
AOAC. Official methods of analysis of AOAC international. 20th ed. Gaithersbg: AOAC; 2016.
Bunkar DS, Bharti P, Meena KK, Goyal SK, Paswan VK. Studies on the optimization and development of functional instant kodo millet based porridge mix. Int J Curr Microbiol Appl Sci. 2020;9(9):1462–80. https://doi.org/10.20546/ijcmas.2020.909.186.
Bunkar DS, Anand A, Kumar K, Meena M, Goyal SK, Paswan VK. Development of production technology for preparation of beetroot powder using different drying methods. Ann Phytomed. 2020;9(2):293–301. https://doi.org/10.21276/ap.2020.9.2.29.
Surve VD, Annapure U. Preparation and characterization of Kharode/Rabadi from fermented pearl millet flour. J Pharmacogn Phytochem. 2019;8(1):756–63.
Ahlawat A, Basak S, Ananthanarayan L. Optimization of spray-dried probiotic buttermilk powder using response surface methodology and evaluation of its shelf stability. J Food Process Preserv. 2022. https://doi.org/10.1111/jfpp.16928.
Vivek K, Mishra S, Pradhan RC. Characterization of spray dried probiotic sohiong fruit powder with Lactobacillus plantarum. LWT-Food Sci Technol. 2020;117(1): 108699. https://doi.org/10.1016/j.lwt.2019.108699.
Elkot WF, Khalil OSF. Physicochemical, textural, microbiological and sensory properties of low-fat bio-labneh using sweet lupine powder and Bifidobacterium longum ATCC 15707. J Food Process Preserv. 2022;46(3): e16311. https://doi.org/10.1111/jfpp.16311.
Arepally D, Reddy RS, Goswami TK. Studies on survivability, storage stability of encapsulated spray dried probiotic powder. Curr Res Food Sci. 2020;3:235–42. https://doi.org/10.1016/j.crfs.2020.09.001.
Seth D, Mishra HN, Deka SC. Functional and reconstitution properties of spray-dried sweetened yogurt powder as influenced by processing conditions. Int J Food Prop. 2017;20(7):1603–11. https://doi.org/10.1080/10942912.2016.1214965.
Tan SL, Sulaiman R, Rukayadi Y, Ramli NS. Physical, chemical, microbiological properties and shelf life kinetic of spray-dried cantaloupe juice powder during storage. LWT-Food Sci Technol. 2021;140(2):110597. https://doi.org/10.1016/j.lwt.2020.110597.
Meena KK, Taneja NK, Jain D, Ojha A, Kumawat D, Mishra V. In vitro assessment of probiotic and technological properties of lactic acid bacteria isolated from indigenously fermented cereal-based food products. Fermentation. 2022;8(10):529–51. https://doi.org/10.3390/fermentation8100529.
Jiang J, Ma C, Song X, Zeng J, Zhang L, Gong P. Spray drying co-encapsulation of lactic acid bacteria and lipids: a review. Trends Food Sci Technol. 2022. https://doi.org/10.1016/j.tifs.2022.09.010.
Rawat K, Kumari A, Kumar R, Ahlawat P, Sindhu SC. Spray-dried lassi powder: process optimisation using RSM and physicochemical properties during storage at room and refrigerated temperature. Int Dairy J. 2022;131:105374. https://doi.org/10.1016/j.idairyj.2022.105374.
Masum AKM, Chandrapala J, Huppertz T, Adhikari B, Zisu B. Influence of drying temperatures and storage parameters on the physicochemical properties of spray-dried infant milk formula powders. Int Dairy J. 2020;105(2):1–9. https://doi.org/10.1016/j.idairyj.2020.104696.
Nguyen DQ, Nguyen TH, Mounir S, Allaf K. Effect of feed concentration and inlet air temperature on the properties of soymilk powder obtained by spray drying. Dry Technol. 2018;36(7):817–29. https://doi.org/10.1080/07373937.2017.1357040.
Ferrari CC, Germer SPM, Alvim ID, Vissotto FZ, de Aguirre JM. Influence of carrier agents on the physicochemical properties of blackberry powder produced by spray drying. Int J Food Sci Technol. 2012;47(6):1237–45. https://doi.org/10.1111/j.1365-2621.2012.02964.x.
Wang W, Dufour C, Zhou W. Impacts of spray-drying conditions on the physicochemical properties of soy sauce powders using maltodextrin as auxiliary drying carrier. CYTA J Food. 2015;13(4):548–55. https://doi.org/10.1080/19476337.2015.1014430.
Seth D, Mishra HN, Deka SC. Effect of spray drying process conditions on bacteria survival and acetaldehyde retention in sweetened yoghurt powder: an optimization study. J Food Process Eng. 2017. https://doi.org/10.1111/jfpe.12487.
Ho TM, Truong T, Bhandari BR. Methods to characterize the structure of food powders—a review. Biosci Biotechnol Biochem. 2017;81(4):651–71. https://doi.org/10.1080/09168451.2016.1274643.
Bhushan B, Sakhare SM, Narayan KS, Kumari M, Mishra V, Dicks LMT. Characterization of riboflavin-producing strains of Lactobacillus plantarum as potential probiotic candidate through in vitro assessment and principal component analysis. Probiotics Antimicrob Prot. 2021;13(2):453–67. https://doi.org/10.1007/s12602-020-09696-x.
Valero-Cases E, Cerdá-Bernad D, Pastor JJ, Frutos MJ. Non-dairy fermented beverages as potential carriers to ensure probiotics, prebiotics, and bioactive compounds arrival to the gut and their health benefits. Nutrients. 2020;12(6):1–27. https://doi.org/10.3390/nu12061666.
Acknowledgements
The authors acknowledge National Institute of Food technology and Entrepreneurship Management, Sonepat, India and Maharana Pratap University of Agriculture and Technology, Udaipur, India for providing necessary facilities during research.
Funding
The external funding was not received for the research.
Author information
Authors and Affiliations
Contributions
Conceptualization: Kamalesh Kumar Meena, Neetu Taneja, Devendra Jain, and Ankur Ojha.; Methodology: Kamalesh Kumar Meena, Ankur Ojha; Formal analysis: Kamalesh Kumar Meena; Writing—original draft preparation, Kamalesh Kumar Meena; writing—review and editing: Kamalesh Kumar Meena, Neetu Taneja, Devendra Jain, and Ankur Ojha. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
This is to certify that the research study titled ‘Development of maize-based Rabaadi powder using probiotic lactic acid bacteria,” involving sensory evaluation by 30 trained and untrained panellists (students and faculty), has been conducted at the Department of Dairy and Food Microbiology, College of Dairy and Food Technology, Maharana Pratap University of Agriculture and Technology, Udaipur, India. Please note that our institution does not have a specific institutional committee dedicated to the approval of sensory studies. However, all procedures outlined in the manuscript have been performed in compliance with our institutional guidelines and policies regarding research ethics. Informed consent was obtained from all individual participants included in the study. Furthermore, it is acknowledged that the authors have confirmed that our institution has been permitted to conduct sensory panel research. While it may not be customary for our institution to have a formal permission process for such research, this information has been duly addressed in place of the permission statement, as recommended. This certificate signifies the adherence of the research study conducted by Dr Kamalesh Kumar Meena and his team to ethical principles and institutional guidelines.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Meena, K.K., Taneja, N.K., Jain, D. et al. Optimization and production of dairy-cereal grain-based probiotic beverage powder and its probiotic survivability under simulated gastric and pancreatic conditions. Discov Food 4, 30 (2024). https://doi.org/10.1007/s44187-024-00099-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44187-024-00099-3