Abstract
In this study, nano and fermented-nano powders of wheat and rice by-products were prepared by superfine grinding of raw and solid-state fermented materials. Effects of fermentation and superfine grinding on phytochemical content, phenolic acids profile as well as antioxidant and anticancer activity were investigated. The results revealed that, phenolic contents of fermented-nano wheat bran (FNWB), fermented-nano wheat germ (FNWG) and fermented-nano rice bran (FNRB) increased by 40.5, 59.2 and 27.9%, respectively compared to their raw samples. The free, conjugated and bound forms of most identified phenolic acids apparently increased. Also, the antioxidant activity of nano and fermented-nano forms significantly increased compared to its raw materials. The anticancer activity of nano and fermented-nano materials against human colon cancer cell line (HCT 116) increased compared to its raw materials. Nano rice bran (NRB) extract was the most effective one with IC50 value of 4.10 mg/mL under the investigated condition. The obtained results indicated that superfine grinding and solid-state fermentation could change the rigid microstructure and liberate bioactive compounds, which enhanced their bio-accessibility and bioavailability to improve bran functionality and usability.
Similar content being viewed by others
1 Introduction
Cereal contains significant levels of dietary antioxidants including phenolic acids, flavonoids, tocopherols and carotenoids [1,2,3]. The most abundant phenolic acids in cereals belong to the chemical class of hydroxycinnamic acids. Ferulic, vanillic, and p-coumaric were major phenolic acids in cereal brans, along with other free phenolic acids including caffeic, chlorogenic, gentisic, syringic, and p-hydroxybenzoic acids [4,5,6]. Phenolic acids may occur in the free form, but are mostly glycoylated with different sugars, especially glucose [7]. In this concern, Vichapong et al. [8], Zilic et al. [9] and Mahmoud et al. [10] reported that wheat bran, wheat germ and rice bran are a source of phytonutrients with potential health benefits, but the nutritional properties will only be fully exploited if whole-meal products are available. Potentially health beneficial compounds such as phenolics, carotenoids and tocopherols are concentrated in the bran layers and germ.
Qu et al. [11] and Liu et al. [12] studied the protective effect of wheat bran against colon and prostate cancer. They found that wheat bran can offer protection against tumor development even when they are consumed with a high-fat diet. Also, Mueller and Voigt [13] and Saiko et al. [14] studied the anti-tumor activity of wheat germ and fermented wheat germ extracts. They reported that wheat germ is a multi-substance composition and, besides others, contains agglutinin, 2-methoxy benzoquinone and 2, 6-dimethoxy benzoquinone which are interact with prostate, human pancreatic and colon-cancer cells. Kong et al. [15] and Takashima et al. [16] stated that rice bran extracts have prominent in vitro growth inhibition on leukaemia tumour cell and human colorectal adenocarcinoma. Leardkamolkarn et al. [17] attributed the potential anti-cancer activity of rice bran in human cancer cell line to the apoptotic induction pathway of pro-apoptotic p53, caspase-3, and cyclin proteins. Chung et al. [18] reported that Lactobacillus rhamnosus and Saccharomyces cerevisiae fermented rice bran inhibited the melanogenesis through downregulation of microphthalmia-associated transcription factor, along with reduced cytotoxicity.
Despite cereal bran are natural, cheap and available phytonutrients sources, they have reduced bioavailability due to their structural position being esterified or covalently bound to arabinoxylan. Regarding this limitation, bioprocessing via solid-state fermentation is gaining popularity. The increase of phenolic compound in fermented samples may be due to the effect of certain enzymes, such as β-glucosidases, esterases and glycoside hydrolase [19]. In this context, Saccharomyces cerevisiae is an economic and safe solution for industrial production. S. cerevisiae was found to produce enzymes like β-glucosidases, carboxylesterases, and possibly feruloyl esterases [20,21,22]. The potential of S. cerevisiae fermentation to improve the health beneficial properties of wheat bran, wheat germ and rice bran were evaluated [23,24,25].
Also, the possible use of nanotechnology in food becomes the focus of research in many countries [26]. Ultrafine powders are easier to incorporate into food systems and more available to the body, which would consequently improve the quality of food products and human health [27]. However, so far the use of this technology in dietary fiber processing remains rather limited, probably due to the toughness and polymer nature of dietary fiber and inadequate equipment support [26]. In this concern, ultrafine ball milling was used to decrease the particle size of wheat bran by Hemery et al. [28, 29]. Also, Rosa et al. [30] evaluated the potential of using ultrafine grinding and electrostatic separation methods to improve the bioaccessibility of p-coumaric acid, sinapic acid and ferulic acid from wheat bran-rich breads. It was observed that, finer the bran particles in bran-rich breads, the more bioaccessible the phenolic acids. Only the free and conjugated phenolic acids forms were found to be bioaccessible, and the bioaccessibility of sinapic acid was much higher than that of ferulic acid, due to the higher solubility of the former.
The objective of our study was to apply modern techniques including, solid-yeast treatment and superfine grinding, to produce nano and fermented-nano powders. Also, the effects of these methods on phytochemical solubility and antioxidant activity as well as anticancer activity of tested materials were investigated.
2 Material and methods
2.1 Materials
Wheat bran (WB) and wheat germ (WG) were obtained from North Cairo Flour Mills Company, Egypt. Rice bran (RB) was obtained from Rice Research and Training Centre, Sakha, Kafr El-Sheikh, Egypt.
2.2 Methods
2.2.1 Stabilization of wheat germ and rice bran
Wheat germ and rice bran were stabilized in an air-oven at a temperature of 120 ± 2 °C for 1 min according to Younas et al. [31]. The stabilized wheat germ and rice bran were ground using Moulinex grinder and passed through a 40-mesh and packed in polyethylene bags and stored at −30 °C until use.
2.2.2 Solid-state yeast fermentation
Yeast strain (Saccharomyces cerevisiae FC-620) was obtained from Microbial Chemistry Dept. collection, National Research Centre, Dokki, Cairo, Egypt. The yeast cells were activated, where; a loopful of the culture was transferred to 250 ml Erlenmeyer flask containing 50 ml broth medium (0.3% yeast extract, 0.3% malt extract, 0.5% peptone and 5% sucrose) and incubated at 30 °C for 24 h under shaking condition. Solid-state yeast treatments were carried out according to the method of Moore et al. [23] as follows: 50 ml of yeast preparation (1380 cfu/ml) was mixed with 100 g sample in a sterile conical flask (1000 ml) to begin the solid-state yeast treatment. Flasks were sealed with cotton seals and incubated at 37 °C for 48 h. All treated samples were dried at 50 ± 1 °C for 16 h, ground using Moulinex grinder and passed through a 40-mesh and stored in polyethylene bags at −30 °C for further analysis.
2.2.3 Preparation of raw, nano and fermented-nano materials
The raw and fermented wheat bran, wheat germ and rice bran (ground using Moulinex grinder to pass through a 40-mesh) were further ground using 5 ml zirconium oxide balls and zirconium oxide bowl volume 250 ml in a PM 100 Planetary Ball-mill (Retsch, Germany) as described by Mohammad et al. [32]. Samples (150 g) were ground at 30 Hz frequency for 60 min at room temperature (25 °C).
2.2.4 Transmission electron microscopy
All ground samples were examined with a JEOL JX 1230 technique with micro analyzer probe, Japan. This technique was used to determine the particle size of the investigated samples.
2.2.5 Preparation of successive extracts
Twenty grams of the raw, nano and fermented-nano materials were extracted using petroleum ether, tetrahydrofuran and methanol in succession using soxhlet apparatus according to the methods of Uc and Nair [33] with some modifications. Each extract obtained following extraction step was filtered using filter paper Whatman No 1, dried using rotary evaporator and the yield of each extract was recorded. Different extracts were reconstituted in 10 mL dimethylsulfoxide (DMSO) and stored under nitrogen at -30 °C till further use.
2.2.6 Determination of total phenolic content
The total phenolic content was determined according to the Folin-Ciocalteu procedure [9]. Where, the obtained extract (100 µL) was transferred into a test tube and the volume adjusted to 3.5 mL with distilled water and oxidized with the addition of 250 µL of Folin–Ciocalteau reagent. After 5 min, the mixture was neutralized with 1.25 mL of 20% aqueous sodium carbonate (Na2CO3) solution. After 40 min, the absorbance was measured at 725 nm against the solvent blank using spectrophotometer (JASCO Model V-530, Japan). The total phenolic content was calculated and expressed as milligrams of gallic acid equivalent (mg GAE) per g of sample based on the calibration curve for absorption of serial gallic acid concentrations (50–500 µg/mL) with regression equation (y = 0.024x + 0.018, R2 = 0.998).
2.2.7 Determination of total flavonoid content
The total flavonoid content was determined according to Zilic et al. [9] using aluminum chloride (AlCl3) colorimetric assay. Where, 300 µL of 5% sodium nitrite (NaNO2) was mixed with 100 µL of extract. After 6 min, 300 µL of a 10% AlCl3 solution was added and the volume was adjusted to 2.5 mL using distilled water. After 7 min, 1.5 mL of 1 M NaOH was added, and the mixture was centrifuged at 5000 g for 10 min. Absorbance of the supernatant was measured at 510 nm against the solvent blank using spectrophotometer (JASCO Model V-530, Japan). The total flavonoid content was determined by means of a calibration curve prepared with catachin, and expressed as milligrams of catechin equivalent (mg CE) per g of sample based on the calibration curve for absorption of serial catechin concentrations (0.1–1 mg/mL) with regression equation (y = 0.008x + 0.043, R2 = 0.999).
2.2.8 Determination of total carotenoids
Total carotenoids content was determined according to Moore et al. [34]. About 100 mg extract were reconstituted in 5 mL methanol. Additionally, 1 mL of 30% methanolic potassium hydroxide (KOH) was added. After vortexing (1 min), mixing, and incubation for 15 min. Carotenoids were extracted with 8 mL of a mixture of hexane:acetone (1:1). The extraction step was repeated twice and organic fractions were combined. To the combined extracts, 25 mL of saturated aqueous sodium chloride solution was added and the mixture was shaken. The hexane phase was transferred into a 50 mL round flask, and the lower aqueous phase was re-extracted with 8 mL of hexane and combined with the 1st extract. Hexane extracts were evaporated to dryness and re-dissolved in acetone and the absorption of each extract was measured directly at 470 nm in duplicate using spectrophotometer (JASCO Model V-530, Japan). Total carotenoids content was calculated based on the calibration curve prepared using serial concentrations of β-carotene standard (25–200 µg/mL) with regression equation (y = 0.007x - 0.02, R2 = 0.999) and expressed as mg β-carotene equivalent (BE) in 100 g extract.
2.2.9 Determination of phenolic acids profile
Free, conjugated and bound Phenolic acids of raw, nano and fermented-nano materials were extracted according to Moore et al. [34] with some modifications as follow: each sample (1 g) was transferred to an Erlenmeyer flask, defatted twice with hexane at a 40:1 ratio (v/w), and kept on a mechanical shaker for 1 h. Each time, the mixture was filtered through a filter paper Whatman No. 1, defatted samples were dried in a hood. The defatted sample was then extracted twice with 80% methanol at a 50:1 ratio (v/w) for 1 h. The mixture was filtered through a filter paper Whatman No. 1, and the combined supernatant was concentrated to dryness using a rotary evaporator at 40 °C. The residue of each sample, that obtained after rotary evaporating, was re-dissolved in 10 mL acidified water with HCl (pH 2) and partitioned with 30 mL of ethyl ether:ethyl acetate (1:1) in a separating funnel, three times. The organic layers contained free phenolic acids were combined and concentrated to dryness using a rotary evaporator at 40 °C and reconstituted in 2 mL methanol. The water phase was neutralized to pH 7 with 2 M sodium hydroxide (NaOH) and dried using a vacuum oven at 50 °C overnight. The residue was dissolved in 10 mL of 2 M NaOH and stirred for 4 h at room temperature (25 °C). The solution was then acidified to pH 2 with 6 M HCl, and extracted three times with ethyl ether and ethyl acetate (1:1). The resulting organic layers contained conjugated phenolic acids were combined, then concentrated to dryness using a rotary evaporator at 40 °C and reconstituted in 2 mL methanol. The residue was hydrolyzed by 40 mL of 2 M NaOH and stirred for 4 h at room temperature (25 °C). The solution was then acidified to pH 2 with 6 M HCl, and extracted three times with ethyl ether and ethyl acetate (1:1). The resulting organic layers contained bound phenolic acids were combined and concentrated to dryness using a rotary evaporator at 40 °C and reconstituted in 2 mL methanol.
HPLC analysis was carried out according to Kim et al. [35] with slight modifications using an Agilent Technologies 1100 series liquid chromatograph equipped with an auto sampler and a diode-array detector. The analytical column was Agilent Eclipse XDB C18 (150 × 4.6 µm; 5 µm) with a C18 guard column. The mobile phase consisted of acetonitrile (solvent A) and 2% acetic acid in water (v/v) (solvent B). The flow rate was kept at 0.8 mL min−1 for a total run time (70 min), and the gradient program was as follows: 100% B to 85% B in 30 min, 85% B to 50% B in 20 min, 50% B to 0% B in 5 min and 0% B to 100% B in 5 min. There was 10 min of post-run for reconditioning. The injection volume was 10 µL and peaks were monitored simultaneously at 280 and 320 nm for the benzoic acid and cinnamic acid derivatives, respectively. All samples were filtered through a 0.45 µm Acrodisc syringe filter (Gelman Laboratory, MI) before injection. Peaks were identified by congruent retention times and UV spectrum and compared with those of the standards.
2.2.10 Determination of DPPH radical scavenging activity
Free radical scavenging capacity of extracts were determined using the stable DPPH• according to Hwang and Nhuan [36]. The final concentration was 200 µM for DPPH• and the final reaction volume was 3.0 mL. The absorbance was measured at 517 nm against a blank of pure methanol after 60 min of incubation in a dark condition using spectrophotometer (JASCO Model V-530, Japan). Percent inhibition of the DPPH free radical was calculated by the following equation:
where:
Acontrol is the absorbance of the control reaction (containing all reagents except the test compound).
Asample is the absorbance with the test compound.
Extract concentration of sample providing 50% inhibition (IC50) was calculated using linear regression analysis.
2.2.11 Determination of ABTS radical scavenging activity
The stock solutions of ABTS•+ reagent was prepared according to Hwang and Nhuan [36] by reacting equal quantities of a 7 mM aqueous solution of ABTS•+ with 2.45 mM potassium persulfate for 16 h at room temperature (25 °C) in the dark. The working solution was then prepared by diluting 1 mL ABTS•+ solution with 60 mL of ethanol: water (50:50, v/v) to obtain an absorbance of 1.0 ± 0.02 units at 734 nm using the spectrophotometer. Extracts (50 µL) were allowed to react with 4.95 mL of the ABTS•+ solution for 1 h in a dark condition. Then the absorbance was taken at 734 nm using spectrophotometer (JASCO Model V-530, Japan). ABTS radical scavenging activity was calculated based on the calibration curve for percent inhibition of serial Trolox concentrations (0.1–1 µM/mL) with regression equation (y = 0.863x – 9.509, R2 = 0.998) and the results were expressed as mM Trolox equivalents (TE)/g sample.
2.2.12 Ferric reducing activity power (FRAP) assay
The FRAP assay was done according to according to Hwang and Nhuan [36]. The stock solutions included 300 mM acetate buffer [3.1 g sodium acetate (C2H3NaO2.3H2O) and 16 mL glacial acetic acid (C2H4O2), pH 3.6], 10 mM TPTZ solution in 40 mM HCl, and 20 mM ferric chloride (FeCl3.6H2O) solution. The fresh working solution was prepared by mixing 25 mL acetate buffer, 2.5 mL TPTZ solution, and 2.5 mL FeCl3.6H2O solution and then warmed at 37 °C before using. Extracts (50 µL) were allowed to react with 3.95 mL of the FRAP solution for 30 min in the dark condition. Readings of the colored product [ferrous tripyridyltriazine complex] were taken at 593 nm using spectrophotometer (JASCO Model V-530, Japan). Ferric reducing activity power was calculated based on the calibration curve for absorption of serial Trolox concentrations (50–500 µM/mL) with regression equation (y = 0.021x – 0.052, R2 = 0.999) and the results were expressed as mM Trolox equivalents (TE)/g sample.
2.2.13 Anticancer activity of prepared extracts on human colon cancer cell lines (HCT-116)
Cell viability was assessed by the mitochondrial dependent reduction of yellow MTT to purple formazan in the Bioassay-Cell Culture Laboratory, National Research Centre, Cairo, Egypt, in a sterile area using a Laminar flow cabinet biosafety class II level (Baker, SG403INT, Sanford, ME, USA) according to Mosmann [37]. Human colon cancer cell lines (HCT-116) were suspended in RPMI 1640 medium. The media were supplemented with 1% antibiotic–antimycotic mixture (10,000 U/mL Potassium Penicillin, 10,000 µg/mL Streptomycin Sulfate and 25 µg/mL Amphotericin B), 1% L-glutamine and 10% fetal bovine serum and kept at 37 °C under 5% CO2. DMSO was the vehicle used for dissolution of plant extracts and its final concentration on the cells was less than 0.2%.
2.2.14 Statistical analysis
All samples were analyzed in triplicates and the results were expressed as means ± standard error, except successive extraction yield and Phenolic acids profile, n = 1. The significant difference between the mean values were determined by using the analysis of variance (ANOVA) and Duncan’s multiple range test was conducted at a significance level of p < 0.05 using SPSS 11 program.
3 Results and discussion
3.1 Particle size analysis
Raw and fermented wheat bran, wheat germ and rice bran were ground using high-energy nano-ball-milling with ZrO2 balls (5 mm in diameter). After ultrafine milling, Transmission Electron Microscopy (TEM) was used to determine the particle size of the tested materials. The TEM micrographs of these samples are shown in Additional file 1: Fig. S1 (supporting materials). The TEM images give a detailed view of the particle size and morphology of tested materials. The particle size of the wheat bran, wheat germ and rice bran is distributed in a range from 10–21, 7–19 and 15–47 nm, respectively (Additional file 1: Fig. S1), which indicated that they are in the nano-scale. In addition, a limited amount of the particles of fermented materials were found agglomerated. These results reveal that pulverization by high-energy ball-milling can effectively reduce the particles size to a nano-scale; and it is thus feasible to utilize this treatment to manufacture ultrafine powder.
3.2 Successive extraction yields of raw, nano and fermented-nano materials
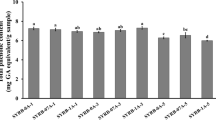
The effect of ultrafine grinding and fermentation of tested materials on the solubility of different types of phytochemical in petroleum ether, tetrahydrofuran and methanol, as well as total yield is illustrated in Fig. 1. The yields of petroleum ether, tetrahydrofuran and methanol extracts were 1.97, 1.07 and 2.74% for WB; 6.85, 1.83 and 15.36% for WG; 2.81, 1.86 and 2.19% for RB, respectively. Similar yields were reported by Oufnac [38] and Wang et al. [39]. They attributed the higher yield of methanol to that methanol solvent possibly extracts not only lipids and small molecule polar compounds, but also some large molecule polar compounds, such as alcohol soluble proteins and carbohydrates. On the other hand, ultrafine grinding increased the yield for both tested materials and solvents. This could be due to increase the surface area of the produced nano-powders of tested materials. Also, fermentation process increased the solubility of tested materials in all solvents, except FNWG. The low yield values of FNWG, especially in methanol, could be explained by consumption of large portion of micro and macro-nutrients during the growth of yeast cells.
Successive extraction yield of raw, nano and fermented-nano Successive extraction yield of raw, nano and fermented-nano materials. WB- wheat bran, NWB- nano-wheat bran, FNWB- fermented-nano- wheat bran, WG- wheat germ, NWG- nano-wheat germ, FNWG- fermented-nano-wheat germ, RB- rice bran, NRB- nano-rice bran, FNRB- fermented-nano-rice bran
3.3 Phytochemical analysis
The results of phytochemical analysis (total phenols, total flavonoids and total carotenoids) conducted on successive extracts of tested materials are presented in Table 1. As shown in this table, total phenolic contents in the investigated samples were the highest in WG, 3.00 mg gallic acid equivalent (GAE) per gram sample. Lower total phenolic contents were present in RB 2.65 mg GAE/g sample) and the lowest in WB 1.66 mg GAE/g sample. A similar phenolic content in wheat bran (1.24 mg GAE/g) and rice bran (2.5 mg GAE/g) had been reported by Zhu et al. [26] and Lai et al. [40]. Ultrafine grinding significantly increased the phenolic contents of NWB and NRB (2.10 and 3.51 mg GAE, respectively) as compared to WB and RB, while this increase in NWG was not significant. Fermentation process did not significantly alter the phenolic content in FNWB or FNRB compared to its nano-forms, while phenolic content of FNWG significantly increased to 4.78 mg GAE/g. Katina et al. [41] reported that the amounts of total phenolic content did not change in rice bran ferments, while Dordevic et al. [24] reported that fermentation of wheat bran by both S. cerevisiae and Lactobacillus rhamnosus increased the phenolic content in wheat extracts. They explained the increase in the total phenolic content by the ability of fungi to degrade lignocellulosic materials due to their highly efficient enzymatic system. Xylanases, in particular, are one type of enzyme missing from S. cerevisiae which are important for release of phenolic compounds from cereal matrix [42]. This could explain the inability of yeast to release the phenolic compounds from wheat and rice matrix. Also, Adebo et al. [43] reported a concurrent reduction in total flavonoids, total tannins and total phenols contents of fermented sorghum. The reported reductions were attributed to degradation and hydrolysis of these compounds during fermentation process.
Also, data in Table 1 showed that the total flavonoids content of WG (2.64 mg catachin equivalent (CE)/g) was significantly higher than those of WB and RB (0.59 and 1.21 mg CE/g, respectively). Ultrafine grinding significantly increased the flavonoids content of NWB and NRB to 1.26 and 1.81 mg CE/g, respectively, but its effect on NWG was not significant. Moreover, fermentation process significantly increased the flavonoids content of FNWB and FNRB (2.04 and 2.57 mg CE/g, respectively). Also, the effect of fermentation process on FNWG flavonoids content was not significant. Similar results were reported by Zilic et al. [9] for wheat genotypes and El Bedawey et al. [44] for wheat germ and rice bran. Brewer et al. [45] compared the flavonoids content of coarse, medium and fine wheat bran from the same wheat cultivar. The order of flavonoid content was determined as: fine > coarse ~ medium. Prabhu et al. [25] mentioned that fermentation of rice bran by yeast resulted about 14% and 18% increase in flavonoid content after 24 and 48 h of fermentation. This was attributed to the increase in acidic value during fermentation that is liberating bound flavonoid components and making it more bioavailable.
Total carotenoids contents of investigated samples ranged from 1.02 to 3.58 mg β-carotene equivalent (βCE)/g (Table 1). Among the tested raw materials WG had the highest total carotenoids content (1.984 mg βCE/g). There were no significant differences between the carotenoids contents of WB and RB (1.05 and 1.26 mg βCE/g, respectively). Ultrafine grinding significantly increased the carotenoids contents of NWG and NRB to 2.38 and 2.08 mg βCE/g, respectively. This increase in NWB was not significant. Furthermore, fermentation process significantly increased the total carotenoids of FNWG which recorded the highest total carotenoids content (3.58 mg βCE/g) among all tested forms of the investigated materials. Also, the increase in total carotenoids contents of FNWB as a result of fermentation process was not significant. Zilic et al. [9] found that the total yellow pigments in the brans of bread and durum wheat genotypes ranged from 4.66 to 6.62 mg βCE/kg, and from 8.65 to 12.55 mg βCE/kg, respectively.
3.4 Phenolic acids profiles of wheat and rice by-products
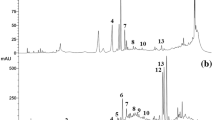
The phenolic acids (gallic, protocatechuic, gentistic, syringic, chlorogenic, caffeic, vanillic, ferulic, sinapic, p-coumaric, rosmarinic, trans-cinnamic acids and chyrsin) which were investigated in cereal by-products and the concentrations of individual phenolic are shown in Table 2 and Additional file 1: Figs S2–S10 (supporting materials). Phenolic acids profile of WB, WG and RB was nearly similar. Among the tested phenolic acids, only gentistic and chlorogenic acids were not detected in WB and WG while, chlorogenic acid was not detected in RB under the experimental conditions. Ferulic and sinapic acids were the predominant phenolic acids in WB and WG while, ferulic and vanillic acids were the predominant phenolic acids in RB. Most of the ferulic and sinapic acids in WB were bound, with a concentration of 129.51 and 80.15 µg/g, respectively. While, the most of ferulic acid in WG was bound, with a concentration of 105.29 µg/g, but most of sinapic acid was conjugated, with a concentration of 127.48 µg/g. Most of the ferulic and vanillic acids in RB were bound, with a concentration of 147.96 and 56.15 µg/g, respectively.
Ultrafine grinding of raw WB and WG releases detectable free and conjugated amounts of gallic and protocatechuic acids. Also, NRB contained detectable free amounts of sinapic, p-coumaric, and rosmarinic acids which were not detected in RB. Moreover, ultrafine grinding of WB, WG and RB apparently increased the free, conjugated and bound forms of all identified phenolic acids except conjugated sinapic acid in WG and RB. This could be due to that ultrafine grinding increased phenolic acids accessibility by increasing the particle surface area of cell walls, and thus increasing the release of intra-cellular contents.
Similar results were obtained by Van Craeyveld et al. [46]. They reported that the intensive grinding of wheat bran could partly solubilize the arabinoxylans, possibly contributing to the production of bioaccessible phenolic compounds, i.e. phenolics which are in conjugated or even free forms. While, Rosa et al. [30] found that the mechanical treatment did not change the phenolic acids structuration state as the conjugated and free forms remained constant among the ground fractions. They mentioned that the conditions of grinding used (frequency and time) probably were not hard enough to break phenolic acids ester link.
On the other hand, the concentrations of soluble free and conjugated gallic, syringic, sinapic, p-coumaric, and rosmarinic acids of fermented nano-samples showed pronounced increases versus its raw and nano-samples. This indicates that yeast may produce hydrolytic enzymes capable of releasing soluble conjugated or insoluble bound phenolic acids from wheat bran. In contrast, soluble free ferulic and vanillic acids concentrations in FNWG and FNRB, respectively showed decreased values compared to NWG and NRB. This decrease indicates that yeast may be able to convert ferulic and vanillic acids to other compounds through enzymatic reactions.
Interestingly, strains of S. cerevisiae have been reported to have a variety of phenolic acid biotransformation activities involving ferulic and vanillic acid derivatives [47]. This may partially explain the observed changes in soluble free phenolics. Furthermore, results showed that fermentation altered soluble conjugated and insoluble bound concentrations for most detected phenolic acids. Fermentation of WB and WG decreased insoluble bound concentrations for all measured phenolic acids versus nano-form, except for ferulic and p-coumaric acids. These results suggest that S. cerevisiae may produce enzymes that capable of releasing insoluble bound phenolic acids, thereby increasing its soluble free and or soluble conjugated phenolic acid contents. On contrast, fermentation of RB increased insoluble bound concentrations of all measured phenolic acids versus NRB. This could be due to the differences in lignocellulosic materials and phenolic acids profile of wheat and rice cultivars. Moore et al. [23] and Chen et al. [48] studied the effect of yeast and fungal fermentation on soluble free, soluble conjugated and insoluble bound phenolic acids of wheat and rice bran, respectively and found similar results.
3.5 Antioxidant activity of raw, nano and fermented-nano materials
The extracts of investigated samples were analyzed and compared for their IC50 values against DPPH• (Table 3). IC50 is the required concentration of sample antioxidants to scavenge 50% DPPH radicals in the reaction mixtures under the experimental conditions. The IC50 values ranged from 1.73 mg for WG to 0.51 mg for NRB, indicating that individual samples may significantly differ in their DPPH• radical scavenging capacities. The scavenging effect against DPPH• radical ranked the samples in the order of rice bran > wheat bran > wheat germ. Scavenging activity of all nano-materials slightly increased compared to its raw materials. Also, the scavenging activity of FNWG increased compared to its nano-forms, while the scavenging activity of FNWB and FNRB decreased. This could be due to the ability of yeast to increase the amount of extracted phytochemicals. These results were in agreement with those of Moore et al. [34], Mansour et al. [49] and Shin et al. [50]. While, Prabhu et al. [25] depicted that the fermented rice bran extract exhibited about 56% radical scavenging activity with 24 h of fermentation. They attributed this enhancement of scavenging activity to the liberation of bound polyphenolic and flavonoid content by the fermentative action of yeast.
All tested samples exhibited effectual radical cation scavenging activity ranged from 4.61 mM trolox equivalent (TE)/g WB to 8.27 mM TE/g NWB, as seen in Table 3. There were no significant differences in ABTS•+ scavenging potential among WB, WG or RB. Ultrafine grinding significantly increased the scavenging activity of NWB and NRB to 8.27 and 8.08 mM TE/g, respectively. Also, the scavenging activity of FNWB and FNRB were significantly higher than those of WB and RB. On the other hand, neither ultrafine grinding nor fermentation significantly affected the scavenging activity of wheat germ. Moore et al. [34] found that soft wheat grains had ABTS•+ scavenging activities varied from 14.3 to 17.6 µM TE/g. Also, wheat bran had 73.24% ABTS radical scavenging activity [51]. Mahmoud et al. [10] mentioned that 1 µg/ml of wheat germ extract had ability to scavenging 70% from the ABTS•+ radicals.
The results of reducing power demonstrate the electron donor properties of tested samples thereby neutralizing free radicals by forming stable products (Table 3). The outcome of the reducing reaction is to terminate the radical chain reactions that may otherwise be very damaging. WB had the lowest reducing power (4.55 mM TE/g). There were no significant differences in reducing power of WG and RB (5.79 and 6.00 mM TE/g, respectively). Ultrafine grinding significantly increased the reducing power of NWB and NRB to 7.60 and 7.40 mM TE/g, respectively. While, the fermentation process only increased the reducing power of FNWG to 6.88 mM TE/g compared to 5.7 mM TE/g for both WG and NWG. Lai et al. [40] found that the antioxidant activity of the methanolic extract of rice bran was 78% of reducing power. Singh et al. [52] reported that the reducing power of Wheat bran was 2.532 mM ascorbic acid equivalent (AAE)/g. In general, the obtained results are comparable to those previously reported by Vrancheva et al. [53].
3.6 Anticancer activity of raw, nano and fermented-nano materials
The effect of successive extracts of tested samples on proliferation of human colon cancer cell line HT-116 was investigated using MTT assay at 4 concentrations (10, 7.5, 5 and 2.5 mg/ml) and IC50 and IC90 were calculated using the probit analysis as shown in Table 4. Among the tested raw materials only RB extract was effective against cancer cell proliferation with IC50 values of 6.47. Cytotoxic activity of WB and WG successive extracts showed a dramatic inhibition drop against cancer cell growth from 63.8 and 82.6% at 10 mg/ml, respectively to 0% at 5 mg/ml. The anticancer activity of ultrafine ground samples increased compared to its raw materials. Also, NRB extract was the most effective treatment with IC50 value of 4.10 mg/ml followed by 7.77 mg/ml for NWG and 14.30 mg/ml for NWB. Also, the extracts of FNWB and FNWG showed lower IC50 values compared to the extracts of raw and nano forms which indicate that fermentation process increased the anticancer activity of these materials. The acceptable explanation for these results can be due the presence of polyphenols that possess anticancer properties by blocking cell cycle progression [54]. In this concern, some identified phenolic acids including p-coumaric, ferulic, and sinapinic acids have been previously shown to inhibit the growth of some cancer cell lines [55, 56]. The antiproliferative activities of p-coumaric, ferulic, and sinapinic acids against HeLa, HCT116, and HT29 cancer cell lines were examined by Senawong et al. [57]. The MTT assay showed that ferulic, sinapinic and p-coumaric acids could inhibit the growth of tumor cells at millimolar concentrations. p-Coumaric acid exhibited the greatest anticancer activity against all tested cancer cell lines. Moreover, rice bran fermented products were found to arrest the cancer cell cycle, promote cancer cell apoptosis and enhance the chemo-preventive effects [58].
3.7 Correlation between antioxidants, antioxidant activity and anticancer activity of raw, nano and fermented-nano materials
Data in Table 5 showed high correlation between the techniques used for determining antioxidant activity. High negative correlations among IC50 determined based on DPPH assay and both ABTS and FRAP assays were found (r = -0.821 and 0.825, respectively, p < 0.01). Also, correlations among ABTS and FRAP assays were positively high (r = 0.997, p < 0.01). Similarly, Connor et al. [59] found high correlation among ORAC, FRAP, and methyl linoleate oxidation assays in blueberries. Awika et al. [60] also found high correlation between ORAC, ABTS, and DPPH in sorghum and its products. Moreover, DPPH, ABTS and FRAP were highly correlated with both total phenols (r = −0.836, 0.998 and 0.992, respectively, p < 0.01) and total flavonoids (r = −0.808, 0.992 and 0.995, respectively, p < 0.01) of the tested materials (Table 5). Whereas, the correlation between antioxidant activity assay methods and total carotenoids was not significant (r = −0.441, 0.238 and 0.286, respectively). Both total phenols and total flavonoids showed high correlation with antioxidant activity as determined by all assays, which indicates that they are important contributors to antioxidant activity in tested extracts. Gil et al. [61] found high correlation (r = 0.9, P < 0.05) between antioxidant activities determined by DPPH or FRAP assays and total phenols.
On the other hand, negative correlation between IC50 values determined in anticancer activity test and both total phenols and total flavonoids (r = −0.527 and −0.555, respectively, P < 0.05), while the correlation between cytotoxic activity and total carotenoids was not significant (r -0.028). This significant correlation (P < 0.05) indicates that phenolic and flavonoid compounds are effective anticancer agents in the tested extracts. Also, there was negative correlation between IC50 values determined in anticancer activity test and both ABTS and FRAP (r = −0.534 and −0.539, respectively, P < 0.05). The highest positive correlation was found between cytotoxic activity and DPPH (r = 0.648, P < 0.01). For this reason, phytochemicals may play a positive role in the induction of cytotoxic effect in the tested cell through its antioxidant activity.
4 Conclusion
This study demonstrates that cereal by-products including wheat bran, wheat germ and rice bran are good sources of dietary antioxidants. High energy ball milling and solid-state fermentation are promising techniques in food processing. These techniques were able to produce nano and fermented-nano powders from cereal by-products with higher antioxidant and anticancer activity through reducing the particle sizes and liberating the phytochemicals. High significant correlation (P < 0.05) between total phenols, total flavonoids, antioxidant assays (DPPH, ABTS, FRAP) and anticancer activity, which indicates that phenolic and flavonoid compounds are effective antioxidant and anticancer agents in the tested extracts. Superfine grinding and solid-state fermentation can be involved in cereal processing to valorize the economical and nutritional value of cereal by-products.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Kumar N, Goel N. Phenolic acids: natural versatile molecules with promising therapeutic applications. Biotechnol Rep. 2019;24: e00370. https://doi.org/10.1016/j.btre.2019.e00370.
Przybylska-Balcerek A, Frankowski J, Stuper-Szablewska K. Bioactive compounds in sorghum. Eur Food Res Technol. 2019;245:1075–80. https://doi.org/10.1007/s00217-018-3207-0.
Horvat D, Šimić G, Drezner G, Lalić A, Ledenčan T, Tucak M, Zdunić Z. Phenolic acid profiles and antioxidant activity of major cereal crops. Antioxidants. 2020;9:527. https://doi.org/10.3390/antiox9060527.
Sun MS, Mihyang K, Song JB. Cytotoxicity and quinine reductase induced effects of Daucas carrot leaf extracts on human cancer cells. Kor J Food Sci. 2001;30:86–91.
Adom KK, Sorrells ME, Liu RH. Phytochemical profiles and antioxidant activity of wheat varieties. J Agric Food Chem. 2003;51:7825–34. https://doi.org/10.1021/jf030404l.
Zhou K, Laux JJ, Yu L. Comparison of Swiss red wheat grain and fractions for their antioxidant properties. J Agric Food Chem. 2004;52:1118–23. https://doi.org/10.1021/jf030640w.
Chandrasekara A. Phenolic Acids. Encyclopedia of food chemistry. Elsevier: Amsterdam; 2019.
Vichapong J, Sookserm M, Srijesdaruk V, Swatsitang P, Srijaranai S. High performance liquid chromatographic analysis of phenolic compounds and their antioxidant activities in rice varieties. LWT-Food Sci Technol. 2010;43:1325–30. https://doi.org/10.1016/j.lwt.2010.05.007.
Zilic S, Serpen A, Akıllıoğlu G, Janković M, Gökmen V. Distributions of phenolic compounds, yellow pigments and oxidative enzymes in wheat grains and their relation to antioxidant capacity of bran and debranned flour. J Cereal Sci. 2012;56:652–8. https://doi.org/10.1016/j.jcs.2012.07.014.
Mahmoud AA, Mohdaly AAA, Elneairy NAA. Wheat germ: an overview on nutritional value, antioxidant potential and antibacterial characteristics. Food Nutr Sci. 2015;06:265–77. https://doi.org/10.4236/fns.2015.62027.
Qu H, Madl RL, Takemoto DJ, Baybutt RC, Wang W. Lignans are involved in the antitumor activity of wheat bran in colon cancer SW480 cells. J Nutr. 2005;135:598–602. https://doi.org/10.1093/jn/135.3.598.
Liu L, Winter KM, Stevenson L, Morris C, Leach DN. Wheat bran lipophilic compounds with in vitro anticancer effects. Food Chem. 2012;130:156–64. https://doi.org/10.1016/j.foodchem.2011.07.023.
Mueller T, Voigt W. Fermented wheat germ extract-nutritional supplement or anticancer drug? Nutr J. 2011;10:1–6. https://doi.org/10.1186/1475-2891-10-89.
Saiko P, Ozsvar-Kozma M, Graser G, Lackner A, Grusch M, Madlener S, Szekeres T. Avemar, a nontoxic fermented wheat germ extract, attenuates the growth of sensitive and 5-FdUrd/Ara-C cross-resistant H9 human lymphoma cells through induction of apoptosis. Oncol Rep. 2009;21:787–91. https://doi.org/10.3892/or_00000285.
Kong CKL, Lam WS, Chiu LCM, Ooi VEC, Sun SSM, Wong YS. A rice bran polyphenol, cycloartenyl ferulate, elicits apoptosis in human colorectal adenocarcinoma SW480 and sensitizes metastatic SW620 cells to TRAIL-induced apoptosis. Biochem Pharmacol. 2009;77:1487–96. https://doi.org/10.1016/j.bcp.2009.02.008.
Takashima A, Ohtomo M, Kikuchi T, Iwashita J, Abe T, Hata K. Differentiation-and apoptosis-inducing activities of rice bran extracts in a human colon cancer cell line. J Food Sci Technol. 2013;50:595–9. https://doi.org/10.1007/s13197-011-0368-2.
Leardkamolkarn V, Thongthep W, Suttiarporn P, Kongkachuichai R, Wongpornchai S, Wanavijitr A. Chemopreventive properties of the bran extracted from a newly-developed thai rice: the riceberry. Food Chem. 2011;25:978–85. https://doi.org/10.1016/j.foodchem.2010.09.093.
Chung SY, Seo YK, Park JM, Seo MJ, Park JK, Kim JW, Park CS. Fermented rice bran downregulates MITF expression and leads to inhibition of α-MSH-induced melanogenesis in B16F1 Melanoma. Biosci Biotechnol Biochem. 2009;73:1704–10. https://doi.org/10.1271/bbb.80766.
Călinoiu LF, Cătoi AF, Vodnar DC. Solid-state yeast fermented wheat and oat bran as a route for delivery of antioxidants. Antioxidants. 2019;8:372–90. https://doi.org/10.3390/antiox8090372.
Lomolino G, Lante A, Crapisi A, Spettoli P, Curioni A. Detection of Saccharomyces cerevisiae carboxylesterase activity after native and sodium dodecyl sulfate electrophoresis by using fluorescein diacetate as substrate. Elect. 2001;22:1021–3. https://doi.org/10.1002/1522-2683()22:6%3c1021::AID-ELPS1021%3e3.0.CO;2-B.
Hernández LF, Espinosa JC, Fernández-González M, Briones A. β-glucosidase activity in a Saccharomyces cerevisiae wine strain. Int J Food Microbiol. 2003;80:171–6. https://doi.org/10.1016/s0168-1605(02)00149-6.
Coghe S, Benoot K, Delvaux F, Vanderhaegen B, Delvaux FR. Ferulic acid release and 4-vinylguaiacol formation during brewing and fermentation: indications for feruloyl esterase activity in Saccharomyces cerevisiae. J Agric Food Chem. 2004;52:602–8. https://doi.org/10.1021/jf0346556.
Moore J, Cheng Z, Hao J, Guo G, Liu JG, Lin C, Yu LL. Effects of solid-state yeast treatment on the antioxidant properties and protein and fiber compositions of common hard wheat bran. J Agric Food Chem. 2007;55:10173–82. https://doi.org/10.1021/jf071590o.
Dordevic TM, Šiler-Marinković SS, Dimitrijević-Branković SI. Effect of fermentation on antioxidant properties of some cereals and pseudo cereals. Food Chem. 2010;119:957–63. https://doi.org/10.1016/j.foodchem.2009.07.049.
Prabhu AA, Mrudula CM, Rajesh J. Effect of yeast fermentation on nutraceutical and antioxidant properties of rice bran. Int J Agric Food Sci. 2014;4:59–65.
Zhu K, Huang S, Peng W, Qian H, Zhou H. Effect of ultrafine grinding on hydration and antioxidant properties of wheat bran dietary fiber. Food Res Int. 2010;43:943–8. https://doi.org/10.1016/j.foodres.2010.01.005.
Raghavendra SN, Rastogi NK, Raghavarao KSMS, Tharanathan RN. Dietary fiber from coconut residue: effects of different treatments and particle size on the hydration properties. Eur Food Res Technol. 2004;218:563–7. https://doi.org/10.1007/s00217-004-0889-2.
Hemery YM, Anson NM, Havenaar R, Haenen GRMM, Noort MWJ, Rouau X. Dry-fractionation of wheat bran increases the bioaccessibility of phenolic acids in breads made from processed bran fractions. Food Res Int. 2010;43:1429–38. https://doi.org/10.1016/j.foodres.2010.04.013.
Hemery Y, Chaurand M, Holopainen U, Lampi AM, Lehtinen P, Piironen V, Rouau X. Potential of dry fractionation of wheat bran for the development of food ingredients, part I: Influence of ultra-fine grinding. J Cereal Sci. 2011;53:1–8. https://doi.org/10.1016/j.jcs.2010.09.005.
Rosa NN, Barron C, Gaiani C, Dufour C, Micard V. Ultra-fine grinding increases the antioxidant capacity of wheat bran. J Cereal Sci. 2013;57:84–90. https://doi.org/10.1016/j.jcs.2012.10.002.
Younas A, Bhatti MS, Ahmed A, Randhawa MA. Effect of rice bran supplementation on cookie baking quality. Pak J Agric Sci. 2011;48:129–34.
Mohammad AA, Yousif EI, Yaseen AA, Gdallah MG, Shouk AA, Abdel Fatah AA. Physico-chemical and functional properties of nano and fermented-nano powders of some food plant by-products. Cur Sci Int. 2015;4:503–14.
Uc R, Nair VMG. Phytochemical analysis of successive reextracts of the leaves of Moringa oleifera Lam. Int J Pharm Pharm Sci. 2013;5:629–34.
Moore J, Hao Z, Zhou K, Luther M, Costa J, Yu L. Carotenoid, tocopherol, phenolic acid, and antioxidant properties of Maryland-grown soft wheat. J Agric and Food Chem. 2005;53:6649–57. https://doi.org/10.1021/jf050481b.
Kim K, Tsao R, Yang R, Cui S. Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effect of hydrolysis conditions. Food Chem. 2006;95:466–73. https://doi.org/10.1016/j.foodchem.2005.01.032.
Hwang ES, Nhuan DT. Effects of extraction and processing methods on antioxidant compound contents and radical scavenging activities of laver (Porphyra tenera). Prev Nutr Food Sci. 2014;19:40–8. https://doi.org/10.3746/pnf.2014.19.1.040.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. https://doi.org/10.1016/0022-1759(83)90303-4.
Oufnac DS. Determination of Antioxidant Capacity in Corn Germ, Wheat Germ and Wheat Bran Using Solvent and Microwave-Assisted Solvent Extraction. LSU MSc Theses 2006; https://digitalcommons.lsu.edu/gradschool_theses/545
Wang WM, Klopfenstein CF, Ponte JG. Effects of twin-screw extrusion on the physical properties of dietary fiber and other components of whole wheat and wheat bran and on the baking quality of the wheat bran. Cereal chem. 1993;70:707–11.
Lai P, Li KY, Lu S, Chen HH. Phytochemicals and antioxidant properties of solvent extracts from Japonica rice bran. Food Chem. 2009;117:538–44. https://doi.org/10.1016/j.foodchem.2009.04.031.
Katina K, Juvonen R, Laitila A, Flander L, Nordlund E, Kariluoto S, Poutanen K. Fermented wheat bran as a functional ingredient in baking. Cereal Chem J. 2012;89:126–34. https://doi.org/10.1094/cchem-08-11-0106.
Mathew S, Abraham TE. Ferulic acid: an antioxidant found naturally in plant cell walls and feruloyl esterases involved in its release and their applications. Crit Rev Biotechnol. 2004;24:59–83. https://doi.org/10.1080/07388550490491467.
Adebo OA, Njobeh PB, Adebiyi JA, Kayitesi E. Co-influence of fermentation time and temperature on physicochemical properties, bioactive components and microstructure of ting (a Southern African food) from whole grain sorghum. Food Biosci. 2018;25:118–27. https://doi.org/10.1016/j.fbio.2018.08.007.
El Bedawey AAE, Mansour EH, Zaky MS, Hassan AA. Characteristics of antioxidant isolated from some plant sources. Food Nutr Sci. 2010;01:5–12. https://doi.org/10.4236/fns.2010.11002.
Van Craeyveld V, Holopainen U, Selinheimo E, Poutanen K, Delcour JA, Courtin CM. Extensive dry ball milling of wheat and rye bran leads to in situ production of arabinoxylan oligosaccharides through nanoscale fragmentation. J Agric Food Chem. 2009;57:8467–73. https://doi.org/10.1021/jf901870r.
Brewer LR, Kubola J, Siriamornpun S, Herald TJ, Shi YC. Wheat bran particle size influence on phytochemical extractability and antioxidant properties. Food Chem. 2014;152:483–90. https://doi.org/10.1016/j.foodchem.2013.11.128.
Priefert H, Rabenhorst J, Steinbüchel A. Biotechnological production of vanillin. Appl Microbiol Biotechnol. 2001;56:296–314. https://doi.org/10.1007/s002530100687.
Chen Y, Ma Y, Dong L, Jia X, Liu L, Huang F, Zhang R. Extrusion and fungal fermentation change the profile and antioxidant activity of free and bound phenolics in rice bran together with the phenolic bioaccessibility. LWT. 2019;115: 108461. https://doi.org/10.1016/j.lwt.2019.108461.
Mansour E, Khaled AB, Lachiheb B, Abid M, Bachar K, Ferchichi A. Phenolic compounds, antioxidant, and antibacterial activities of peel extract from Tunisian pomegranate. J Agric Sci Technol. 2013;15:1393–403.
Shin HY, Kim SM, Lee JH, Lim ST. Solid-state fermentation of black rice bran with Aspergillus awamori and Aspergillus oryzae: effects on phenolic acid composition and antioxidant activity of bran extracts. Food chem. 2019;272:235–41. https://doi.org/10.1016/j.foodchem.2018.07.174.
Shallan MAAM, Fayed SAEKS, Gazzar MMEAE. Protective effects of wheat bran and buckwheat hull extracts against hypercholesterolemia in male rats. Int J Adv Res. 2014;2:724–36.
Singh B, Sharma HK, Sarkar BC. Optimization of extraction of antioxidants from wheat bran (Triticum spp.) using response surface methodology. J Food Sci Technol. 2012;49:294–308. https://doi.org/10.1007/s13197-011-0276-5.
Vrancheva R, Popova A, Mihaylova D, Krastanov A. Phytochemical analysis, in vitro antioxidant activity and germination capability of selected grains and seeds. Jordan J Biol Sci. 2020;13:337–42.
AL-Asady AAB, Khalil KH, Barwari SSM. Cytotoxic and cytogenetics effects of aqueous, methanolic and secondary metabolites extracts of Capparis spinosa on tumor cell lines In vitro. Jordan J Biol Sci. 2012;5:15–30.
Jaganathan SK. Events associated with apoptotic effect of p-Coumaric acid in HCT-15 colon cancer cells. World J Gastroenterol. 2013;19:7726. https://doi.org/10.3748/wjg.v19.i43.7726.
Peng CC, Chyau CC, Wang HE, Chang CH, Chen KC, Chou KY, Peng RY. Cytotoxicity of ferulic acid on T24 cell line differentiated by different microenvironments. BioMed Rreh Int. 2013. https://doi.org/10.1155/2013/579859.
Senawong T, Khaopha S, Misuna S, Komaikul J, Senawong G, Wongphakham P, Yunchalard S. Phenolic acid composition and anticancer activity against human cancer cell lines of the commercially available fermentation products of Houttuynia cordata. Sci Asia. 2014;40:420–7. https://doi.org/10.2306/scienceasia1513-1874.2014.40.420.
Yu Y, Zhang J, Wang J, Sun B. The anti-cancer activity and potential clinical application of rice bran extracts and fermentation products. RSC Adv. 2019;9:18060–9. https://doi.org/10.1039/c9ra02439e.
Connor AM, Luby JJ, Tong CBS. Variability in antioxidant activity in blueberry and correlations among different antioxidant activity assays. J Am Soc Hortic Sci. 2002;127:238–44. https://doi.org/10.21273/jashs.127.2.238.
Awika JM, Rooney LW, Wu X, Prior RL, Cisneros-Zevallos L. Screening methods to measure antioxidant activity of sorghum (sorghum bicolor) and sorghum products. J Agric Food Chem. 2003;51:6657–62. https://doi.org/10.1021/jf034790i.
Gil MI, Tomás-Barberán FA, Hess-Pierce B, Kader AA. Antioxidant capacities, phenolic compounds, carotenoids, and vitamin c contents of nectarine, peach, and plum cultivars from California. J Agric Food Chem. 2002;50:4976–82. https://doi.org/10.1021/jf020136b.
Acknowledgements
The authors gratefully acknowledge the National Research Center (Egypt) fund (for their financial support to this work Project no. 10090002).
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
E-SY and AY conceived and designed the plane of work. A-HS and A-FA-F conducted the solid-state fermentation and cytotoxicity experiments. MG and AM conducted the superfine grinding, SEM, TP, TF, HPLC and antioxidant experiments. All authors are involved in data analyzing, and writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Additional file 1: Fig. S1.
Transmission electron micrographs of, nano and fermented-nano-powder wheat bran (a,b); wheat germ (c,d); rice bran (e,f). Fig. S2. HPLC chromatograms of free phenolic acids of raw, nano and fermented-nano wheat bran extracts Peak 1, gallic acid; 2, protocatechuic acid; 3, gentisic acid; 4, chlorogenic acid; 5, caffeic acid; 6, vanillic acid; 7, ferulic acid; 8, sinapic acid; 9, p-coumaric acid; 10, rosmarinic acid; 11, cinnamic acid; 12, chyrsin. Fig. S3. HPLC chromatograms of conjugated phenolic acids of raw, nano and fermented-nano wheat bran extracts Peak 1, gallic acid; 2, protocatechuic acid; 3, gentisic acid; 4, chlorogenic acid; 5, caffeic acid; 6, vanillic acid; 7, ferulic acid; 8, sinapic acid; 9, p-coumaric acid; 10, rosmarinic acid; 11, cinnamic acid; 12, chyrsin. Fig. S4. HPLC chromatograms of bound phenolic acids of raw, nano and fermented-nano wheat bran extracts Peak 1, gallic acid; 2, protocatechuic acid; 3, gentisic acid; 4, chlorogenic acid; 5, caffeic acid; 6, vanillic acid; 7, ferulic acid; 8, sinapic acid; 9, p-coumaric acid; 10, rosmarinic acid; 11, cinnamic acid; 12, chyrsin. Fig. S5. HPLC chromatograms of free phenolic acids of raw, nano and fermented-nano wheat germ Peak 1, gallic acid; 2, protocatechuic acid; 3, gentisic acid; 4, chlorogenic acid; 5, caffeic acid; 6, vanillic acid; 7, ferulic acid; 8, sinapic acid; 9, p-coumaric acid; 10, rosmarinic acid; 11, cinnamic acid; 12, chyrsin. Fig. S6. HPLC chromatograms of conjugated phenolic acids of raw, nano and fermented-nano wheat germ. Peak 1, gallic acid; 2, protocatechuic acid; 3, gentisic acid; 4, chlorogenic acid; 5, caffeic acid; 6, vanillic acid; 7, ferulic acid; 8, sinapic acid; 9, p-coumaric acid; 10, rosmarinic acid; 11, cinnamic acid; 12, chyrsin. Fig. S7. HPLC chromatograms of bound phenolic acids of raw, nano and fermented-nano wheat germ Peak 1, gallic acid; 2, protocatechuic acid; 3, gentisic acid; 4, chlorogenic acid; 5, caffeic acid; 6, vanillic acid; 7, ferulic acid; 8, sinapic acid; 9, p-coumaric acid; 10, rosmarinic acid; 11, cinnamic acid; 12, chyrsin. Fig. S8. HPLC chromatograms of free phenolic acids of raw, nano and fermented-nano rice bran Peak 1, gallic acid; 2, protocatechuic acid; 3, gentisic acid; 4, chlorogenic acid; 5, caffeic acid; 6, vanillic acid; 7, ferulic acid; 8, sinapic acid; 9, p-coumaric acid; 10, rosmarinic acid; 11, cinnamic acid; 12, chyrsin. Fig. S9. HPLC chromatograms of conjugated phenolic acids of raw, nano and fermented-nano rice bran Peak 1, gallic acid; 2, protocatechuic acid; 3, gentisic acid; 4, chlorogenic acid; 5, caffeic acid; 6, vanillic acid; 7, ferulic acid; 8, sinapic acid; 9, p-coumaric acid; 10, rosmarinic acid; 11, cinnamic acid; 12, chyrsin. Fig. S10. HPLC chromatograms of bound phenolic acids of raw, nano and fermented-nano rice branPeak 1, gallic acid; 2, protocatechuic acid; 3, gentisic acid; 4, chlorogenic acid; 5, caffeic acid; 6, vanillic acid; 7, ferulic acid; 8, sinapic acid; 9, p-coumaric acid; 10, rosmarinic acid; 11, cinnamic acid; 12, chyrsin
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yousif, ES., Yaseen, A., Abdel-Fatah, AF. et al. Antioxidant and anticancer properties of nano and fermented-nano powders of wheat and rice by-products. Discov Food 2, 33 (2022). https://doi.org/10.1007/s44187-022-00032-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44187-022-00032-6