Abstract
Recently, the trend of many studies to replace synthetic antifungal commonly used during storage with natural antifungal; Plant extracts has been one of the most promising strategies for this purpose. Therefore, this study aimed to evaluate the aqueous extract of the carob pulp (AECP) at 1.5, 2.5, and 5 mg/ml on the growth of some toxigenic Aspergillus species, and its effectiveness as an anti-mycotoxigenic (aflatoxins (AFs) and ochratoxin A (OTA) during storage red peanut kernels (RPKs) for 90 days. The results indicate that the increased concentration of AECP, the area of inhibition zone increased with all treatments. As well, the strain of Aspergillus ochraceus was more affected by AECP than Aspergillus aflatoxiformans followed by Aspergillus parasiticus which recorded (15.7 ± 0.4 mm), (13.4 ± 0.5 mm), and (11.9 ± 0.4 mm) at 5 mg/ml. Respectively. While, the lowest inhibition was shown with Aspergillus flavus at three concentrations of AECP. The impact of AECP on the accumulation of total AFs and OTA during storage of RPKs infected by A. parasiticus and A. ochraceus, respectively, for 90 days. The results showed that the active compounds in the AECP at 5 mg/ml can protect RPKs for 45 days without detecting any amounts of AFs and OTA. After 90 days, the inhibition percentages for the four types of aflatoxins (AFB1, AFG1, AFB2, AFG2) and OTA compared with positive control samples were 85.1, 76.5, 86.5, 84.2, and 87.5%, respectively. Finally, AFB1 recorded the lowest inhibition percentages of all treatments; on the contrary, it was OTA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Aspergillus spp., such as Aspergillus flavus, A. parasiticus, A. niger, and A. ochraceus attack peanut kernels (PK) during growth, harvest, transportation, and storage, these species have acquired an interest by their ability to produce mycotoxins; which include aflatoxins (AFs) and ochratoxin A (OTA) with optimal environmental conditions for temperature and humidity [1, 2]. The four main AFs produced naturally are known as aflatoxin B1 (AFB1), aflatoxin B2 (AFB2), aflatoxin G1 (AFG1), and aflatoxin G2 (AFG2). B and G refer to the blue and green fluorescent colors produced under UV light on Thin Layer Chromatography (TLC) plates [3]. AFs and OTA have caused harm to human and animal health as carcinogenic, mutagenic, teratogenic, nephrotoxic, hepatotoxic, and immunosuppressive effects [4,5,6]. So many countries have established regulatory limits on AFs and OTA in peanut and other agricultural commodities. In Egypt the regulatory limits as recommended by European Commission Regulation (EC) No. 1881/2006 [7]. On the other hand, recently have been focused on using natural antifungal and anti-mycotoxins, due to the studies indicating possible adverse effects that may be related to the consumption of synthetic compounds [8, 9]. Therefore, the use of plant extracts is one of the promising areas in this field. One of these plants is carob (Ceratonia siliqua L.), which grows in Mediterranean areas, carob pulp (CP) has received considerable attention because it has a wide range of many active compounds comprehensive polyphenols, flavonoids, alkaloids, tannins and antioxidant activity, so many countries use carob pods in traditional medicine with a lot of diseases [10,11,12,13]. Carob pulp is used in many forms, including as a drink or syrup, and carob flour that is added to the manufacture of certain products, especially sweets [14,15,16]. But did not study AECP for control of producing AFs and OTA. The present study aimed to evaluate the effect of AECP as antimycotoxigenic (AFs and OTA) during storage RPKs infected by two toxigenic strains (A. parasiticus and A. ochraceus) for 90 days. After evaluation in vitro on growth some Aspergillus spp, and its ability to produce their toxins.
2 Materials and methods
2.1 Carob pods and peanut
The mature carob pods and red peanut kernels (RPKs) were purchased from the local market of Giza (Egypt). The pods were de-seeded to get pulp then ground using electric coffee grinder (TCG-220, Electric Power: 180–220 Watt, china) to a fine powder ready for extraction by water.
2.2 Fungal strains
Three toxigenic fungi strains from Aspergillus spp were used in this study A. flavus (ATCC 28542), A. parasiticus (ATCC 26692), A. ochraceus (ATCC 22947) and A.aflatoxiformans, it isolated from grains by El-Sayed, A. and -Abdelaziz, A; Microbiology Dep., Zagazig University, and deposited to NCBI GenBank with accession # MN093924.
2.3 Chemicals and solvents
Potato Dextrose Agar (PDA) and yeast extract agar were obtained from Sigma- Aldrich, Lyon, France. The AFs mix standard and solvents were purchased from Sigma Chemical Co. (St. Louis, MO). All solvents were of HPLC grade. The water was double distilled with a Millipore water purification system (Bedford, MA).
3 Methods
3.1 Preparation of AECP
Two and half gram (2.5 g) for carob pulp (CP) were mixed with 25 ml of water. Then sonicated for 30 min at 30 °C in an ultrasound bath (Ultrasonic Bath Sonicator Model (K615HTDP) at frequency 40 kHz. The sample was further centrifuged at 10500 RPM, 20 °C for 15 min. The supernatant was kept at −20 °C until analysis [17].
3.2 Determination of total phenolic content (TPC)
The total phenolic content in aqueous extract for carob pulp (CP) was determined using the Folin–Ciocalteau method as follows: Briefly, 1 ml of extract in a volumetric flask was diluted with distilled water to 46 ml. 1 ml of Folin-Ciocalteu reagent was added and the contents of the flask were blended completely. After 3 min, 3 ml of Na2CO3 (2%) was added, then mixture left for 2 h. The absorbance of the mixture was measured at 760 nm. TPC was calibrated as mg Gallic acid equivalents (GAE) 100 g−1 carob pod by the calibration curve of a Gallic acid equivalent (GAE) standard [18].
3.3 Determination of total flavonoid content (TFC)
The TFC was measured according to the method Yan-Hwa et al. (2000) as follows: 1 ml of extract was diluted with 4.3 ml of 80% aqueous ethanol containing 0.1 ml of 10% Al (NO3)3 and 0.1 ml of 1 M aqueous CH3COOK. After 40 min at room temperature, the absorbance was determined spectrophotometrically at 415 nm. The total flavonoid content was measured by us using a calibration curve of a quercetin equivalent (QE) standard [19].
3.4 Quantification of the total alkaloid content (TAC)
Total alkaloid content was determined by using the method developed by [20] which is based on the reaction between alkaloid with bromocresol green (BCG), resulting in a yellow-colored product. This method has the benefits of sensitivity and stability. In brief, 0.1 ml of AECP was mixed with 2 N HCl, and one milliliter of this solution was transferred to a separatory funnel and washed (three times) using 10 ml chloroform. The pH of this solution was adjusted to neutral with 0.1 N NaOH. Then 5 ml of BCG solution and 5 ml of phosphate buffer were added to this solution. After that, the mixture was shaken and the complex formed was extracted with 1, 2, 3, and 4 ml chloroform using vigorous shaking. The extracts were collected in a 10 ml volumetric flask and diluted to volume with chloroform. The absorbance of the complex in chloroform was measured at 470 nm. Alkaloids (mg/ml) = (Abs- 0.048)/0.021 [20].
3.5 Determination of antioxidant activities
Determination of DPPH radical scavenging activity: The antioxidant activity of the extracts, based on the scavenging activity of the stable DPPH free radical was determined by the method described by Lee et al. [21].
3.6 Determination total content of tannins
The condensed tannins were estimated according to Julkunen-Titto. (1985) and expressed as g of catechin equivalents per 100 g of dry weight [22].
3.7 Assay of antifungal activity
The antifungal activity was tested on the three strains of fungi using agar well diffusion technique. The strains were cultivated on PDA slants at 28 °C for 7 days. Spores were harvested by adding 10 ml of sterile distilled water containing 0.05% Tween 20 and scraping the surface of the culture to free the spores. One ml of spore suspension was inoculated into each plate. Wells of 5 mm diameter was made on the PDA surface and filled with the three graduals with three concentrations of (1.5, 2.5 and 5 mg/ml), obtained from diluting the stock solutions were used. Wells containing with (100 µl pure solvent (water) were used as a negative control, while wells containing with Nystatin (1000 Unit/ ml were considered as positive control. The inoculated plates were incubated at 27 °C for 72 h, and then the antifungal activity was assessed by measuring the zone of inhibition (mm). The results average was calculated from at least three replicates for each treatment [23].
3.8 Control of producing AFs and OTA by AECP in YES medium
The yeast extract sucrose (YES) culture medium (2% yeast extract and 15% sucrose/liter distilled water) was used in this experiment, 100 ml of YES was poured into 250 ml Erlenmeyer flask and autoclaved at 121 °C for 15 min, then cooled and inoculated with 1 ml of spore suspension containing 106 spores/ml of A. parasiticus (ATCC 26692) and A. ochraceus both separately, then treated by gradual with concentrations (1.5, 2.5 and 5 mg/ml) from AECP as treatment samples, while Three replications from YES treated by spores of A. parasiticus (ATCC 26692) and A. ochraceus only as positive samples, and flasks were incubated at 28 °C for 7 days. After the end of the incubation period, the AFs were extracted then determined using HPLC as follows: AFs were extracted from the YES medium by chloroform (20 ml twice with 10 ml YES media) then homogenization for 3 min in a separation funnel. Finally, filtration of the chloroform phase, then evaporated to dryness to use with HPLC. The percentage of inhibition was calculated as the following equation.
where: C is mean concentrations of toxins (AFB1, AFB2, AFG1 and AFG2) in the positive samples that inoculated by spores of A. parasiticus only. T is concentrations of the toxin in the sample containing spores of fungus and AECP (treatment sample) [24].
3.9 Extraction and determination of OTA
Ten milliliters of YES medium were filtered, and then extracted with 20 ml of chloroform in separation funnel. The chloroform phase was filtered and concentrated then dryness under nitrogen to dry film; it was dissolved in 1 ml water/acetonitrile (3: 1 v/v) and mixed well by vortex for 30 s. HPLC analysis according to Ammar and El-Desouky. (2016). On the other hand, the percentage of inhibition OTA was calculated according to the above equation [25].
3.10 Control of producing AFs and OTA in (RPKs) infected by toxigenic fungi by AECP
Autoclaved RPKs were used in the following ten (10) experimental groups for each separately (250 gm each): (1) Positive control: RPKs inoculated with a spore suspension of A. parasiticus (ATCC 26692) and A. ochraceus(ATCC 22947) both are separate; Groups 2, 3, and 4 negative controls: RPKs inoculated with AECP only at (1.5, 2.5 and 5 mg/ml) without suspension of strain, respectively; groups 5, 6, and 7 RPKs infected by A. parasiticus (ATCC 26692) spore suspension plus adding 1.5, 2.5 and 5 mg/ml for AECP, respectively; groups 8, 9 and 10 RPKs infected by A. ochraceus(ATCC 22947) spore suspension plus adding 1.5, 2.5 and 5 mg/ml for AECP, respectively. Samples were stored at room temperature for analysis by HPLC to total AFs and OTA at days 15, 30, 45, 60, 75 and 90 [26, 27].
3.11 Statistical analysis
General Linear Model procedure of the SPSS ver. 18 (IBM Corp, NY) was used to statistically analyse. The significance of the differences among treatment groups was determined by Waller–Duncan k-ratio. All statements of significance were depended on the probability of P ≤ 0.05 was considered to be statistically significant. Values represent averages ± standard deviations for triplicate experiments.
4 Results and discussion
4.1 Major phytochemical components of the AECP
Data presented in (Table 1) revealed that the AECP has the high content from TPC (209 ± 3.21 mg/100 g), TFC (16.4 ± 1.18 mg/100 g), TAC (13.27 ± 1.16 mg/100 g), and tannins (5.71 ± 1.31 mg/100 g), which includes many bioactive compounds, the presence of these compounds makes carob (pods) of therapeutic value, as well as, this results are in good agreement with a previous [28, 29]. Content of TPC, TFC, and TAC is affected by genetic, geographic factors, storage settings, soil and methods of analysis [30, 31]. On the other hand, DPPH free radical scavenging activity was 57.3%. These results confirmed by FTIR analysis (Fig. 1) showed many functional groups such presented lowest Transmittance (T) at 3430 cm−1, 1630.5 cm−1 and 1389 cm.−1 (OH stretching, N–H and –C = C-, and-C-H) for alcohols, phenols, alkanes and alkenes [32, 33]. Phenolic compounds in the CP include primarily hydroxybenzoic acids, flavonols, flavan-3-ols and gallotannins. In addition, Gallic acid and its derivatives such as methyl gallate comprise the majority of phenolic acids, as well as, myrecetin, rutin, and catechin are the main components of the ethanolic, methanolic and aqueous extracts. Concerning flavonoid, CP is rich in flavonols such as quercetin, myricetin, kaempferol and their glucosidic derivatives. Quercetin and myricetin rhamnosides are usually the most abundant flavonoids in carob [17, 34, 35]. These compounds have bioactive effects and health benefits in various therapeutic areas, as well as the prevention of certain diseases such as cancer, diabetes and diarrhea. Additionally, carob has antioxidant activities are of particular interest in human cells, so these natural antioxidants can replace the synthetic antioxidants used in foods, which can be harmful components due to their toxicity and carcinogenic effects [36, 37].
4.2 Evaluation of AECP on growth toxigenic Aspergillus fungi
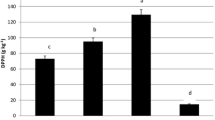
Data presented in (Fig. 2) show the inhibition zone (mm) of fungal growth after treatment with AECP at the concentration 1.5, 2.5 and 5 mg/ml. The obtained data indicated that the highest level of inhibition was detected at the concentration 5 mg/ml of AECP, also, the inhibition increased with increasing the used concentration. In addition the strain of A.ochraceus is more affected by AECP than A. aflatoxiformans. The highest level of inhibition at 5 mg/ml was recorded with A. ochraceus (15.7 ± 0.4 mm) then A. aflatoxiformans (13.4 ± 0.5 mm). Moreover, the lowest inhibition was shown with A. flavus at three treatments. The inhibitory effect of EACP against mycelial growth of Aspergillus ssp. was analyzed statistically as shown in (Table 2). The ANOVA analysis shows significantly both of the concentration EACP and types of fungi. In general, the active compounds in AECP have several mechanisms against fungal, through inhibition of chitin and β-glucan synthesis. In addition, lipid membrane dissolution, and RNA and DNA synthesis alteration. Several previous studies reported that CP contains many compounds phenolics that have antifungal activity such as protocatechuic acid, gallic acid, cinnamic acid and rutin. [38] Reported that the GA was the major phenolic acid present in CP. Moreover, Goulas and Georgiou found that the main components of the water CP extract are myricetin 94.5%, gallic acid 80%, epicatechin 77.3%, and rutin 67% [17, 38]. Which effect on fungal cells through the division and synthesis of RNA leading to inhibition of growth, as kaempferol has been inhibits the expression of protein. Flavones have antifungal activity against pathogenic fungi, may due to Reactive oxygen species (ROS) accumulation is considered one of the primary biochemical causes of apoptosis. Unsuitable regulation of ROS levels can damage, fungal cells, caused to untypical fungal growth and consequential apoptotic-like cell death. As well as, changes in the Mitochondrial membrane like opening of transition pores in the membrane and release of apoptogenic factors into the cytosol, are considered another characteristic of apoptosis [39, 40].
4.3 Inhibitory effect of AECP on the biosynthesis of AFs and OTA
The data presented in (Fig. 3) show the inhibition percentages of AF and OTA produced by A. parasiticus and A. ochraceus, respectively in YES media treated by AECP. The data reflected that the inhibition percentages increased directly with increasing concentration added from AECP. The highest inhibition was recorded with OTA was 18.6, 40.7, 77.8% at (1.5, 2.5, and 5 mg/ml) AECP, at the same concentrations the lowest percentages were 12.8, 22.6 and 61.6% with AFB1. In case YES was treated with 5 mg/ml from AECP the AFG1, AFG2, and AFB2 inhibition were 72.5, 67.3, and 70.05%, respectively. AECP decreased mycelial weight to 63.9% and 54.8% for A. ochraceous and A. parasiticus, respectively at the concentration 2.5 mg/ml (Fig. 4). The phenolic compounds' hydroxyl groups may create hydrogen bonds with some active enzymes, resulting in deactivation and suppression of fungal biomass and the formation of mycotoxins. In addition, AECP causes cytotoxicity in fungi by affecting cell membrane permeability and functions, so that AECP can inactivate biotransformation pathways needed for synthesis AFs and OTA from A. parasiticus and A. ochraceus, respectively [41].
Previous studies used plant extracts to prevent or control the production of AFs; [42] used the crude extracts from mint, sage, bay, anise, and ground red pepper to control the growth of A. parasiticus and its AFs production in vitro. Another study by [43] tested aqueous extracts of fifty-two plants from different families for their antifungal, they found that the potential against eight of Aspergillus spp [42, 43]. Similarly, Pundir and Jain (2010) studied the efficacy of 22 plant extracts against food-associated fungi and found that clove and ginger are more effective than other plant extracts [44]. The alkaloids such as piperine and piperlongumine have an effect on the biosynthesis of OTA -producing from Aspergilli: A. auricomus, A. sclerotiorum, and two isolates of A. alliaceous. The antioxygenic potential of the spices was tested against the OTA-producing strain of A. ochraceus Wilhelm. Clove completely inhibited the mycelial growth of the fungi A. ochraceus, as well as, garlic and laurel completely inhibited the OTA production. Cinnamon and Anis inhibited the synthesis of OTA when used at concentration 3% and mint starting from 4% [45]. Finally, AFs production could be disrupted if any step in the aflatoxin biosynthetic pathway is completely blocked by a specific inhibitor; aflD (nor-1) gene expression that represents the early enzymatic steps in the aflatoxin biosynthetic pathway could be an appropriate target for inhibiting aflatoxin biosynthesis. Disruption or deletion of the aflD (nor-1) gene leads to the accumulation of norsolorinic acid and blocks the synthesis of all aflatoxins and their intermediates beyond norsolorinic acid. In this regard Ren et al. [46], and Buitimea-Cantua et al. [47] reported that the biosynthesis of AFs was inhibited by repression of AFs structural genes which including (aflK and aflLa) may be joint with the down-regulation of the transcription regulator genes (aflR and aflS) in the cluster. Additionally, the suppression of many key AFs genes including aflR, aflD, aflM, and aflP may also be associated with the decreased expression of the global regulator gene veA [46, 47].
4.4 Influence of AECP on accumulation of AFs and OTA during storage of RPKs
This study was carried out to investigate the effect of AECP at 1.5, 2.5, and 5 mg/ml on the accumulation of total AFs and OTA produced by A. parasiticus and A. ochraceus during storage of RPKs for 90 days. Concerning AFs, HPLC analysis did not detect any amount of AFs in infected RPKs by A. parasiticus samples were stored for 15 days after being treated at 1.5, 2.5, and 5 mg/ml for AECP, storage period extended to 45 days with treatment at 5 mg/ml without any amount from AFs (Fig. 5). Whereas extension of the storage causes resulted in an intense accumulation of total AFs in the treated sample and positive control sample both, with differences in concentrations, this is probably due to the increase in the number of spores of A. parasiticus capable of producing toxin. Also, some spores can recover their activity with increasing storage period, and another possibility on the probably for consumption the content of the active compounds in the AECP [48, 49]. The amount of OTA in infected RPKs by A. ochraceus during storage 90 days showed in (Fig. 6). The results indicated that OTA formed after 15, 30, and 45 days from storage after treated RPKs by AECP at 1.5, 2.5, and 5 mg/ml, respectively. On the other hand, the concentration of OTA in the positive control sample was (26.5 µg/kg), while RPKs with 1.5, 2.5, and 5 mg/ml were 21.35, 7.5, and 3.31 µg/kg at the end of the storage period after 90 days. The RPKs treated by AECP at 5 mg/ml and infected by A. ochraceus shown as (Fig. 7). Finally, the percentages of inhibition AFB1, AFG1, AFB2, AFG2, and OTA for each type separately in RPKs treated by AECP after storage for 90 days compared with positive control samples calculated as (Fig. 8). The percentages of inhibition AFB1 in RPKs were lowest percentages with all treatment were 12.3, 50.5, and 76.5% with adding 1.5, 2.5, and 5 mg/ml AECP. On the other hand, OTA had the highest inhibition percentages, which reached 19.3, 71.6, and 87.5% with the same treatments, while AFG1, AFG2 and AFB2 inhibited to 85.1 5, 86.5 and 84.2% with applied 5 mg/ml from AECP.
AFB1 occur more frequently as contaminants, and are also believed to be more potent, than G aflatoxins. From a genomic perspective, the inability to produce G aflatoxins is reported to result from a deletion between the norB (aflF) and cypA (aflU) genes, upstream in the AFs biosynthesis pathway [50]. Previous studies have reported that some phenolic antioxidants, such as gallic, 4-hydroxybenzoic, and chlorogenic acid tended to inhibit OTA production, for example [51], agreement with ([52], [53]) notice a significant reduction in growth rate and OTA production with 250 mg/L of caffeic acid, rutin, and quercetin [51,52,53].
5 Conclusion
In conclusion, this study demonstrated that AECP has the many bioactive compounds (TFC, TPC and TAC), which able to protect of RPKs from toxigenic Aspergillus spp strains. By inhibiting the growth of these strains and forming AFs and OTA during storage RPKs at ambient temperature. The results of the study showed that the AECP at 5 mg/ml is effective and better their antifungal activity against all tested mycotoxigenic fungi, as well against accumulation of AFs and OTA for 45 days of storage of infected RPKs by mycotoxigenic fungi. Whereas RPKs are stored for 90 days, the inhibition percentages of the four types of aflatoxins ranged from 76.5 to 86.5%. However, the percent inhibition of AFB1 was lowest for all treatments. On the other hand, the inhibitory effect increased to 87.5% for OTA. Finally, this is the first study to use AECP in monitoring AFs and OTA during peanut storage. Still necessary are further studies to assess the sensory evaluation, and impact of roasting process after treatment by AECP.
a commercial scale.
Abbreviations
- AFs:
-
Aflatoxins
- AFB1 :
-
Aflatoxin B1
- AFB2 :
-
Aflatoxin B2
- AFG1 :
-
Aflatoxin G1
- AFG2 :
-
Aflatoxin G2
- OTA:
-
Ochratoxin A
- AECP:
-
Aqueous extract of carob pulp
- RPKs:
-
Red peanut kernels
- CP:
-
Carob pulp
- A :
-
Aspergillus
- TPC:
-
Total phenolic content
- TFC:
-
Total flavonoid content
- TAC:
-
Total alkaloid content
References
Dorner JW. Management and prevention of mycotoxins in peanuts. Food Addit Contam. 2008;25(2):203–8. https://doi.org/10.1080/02652030701658357.
Fapohunda SO, Annabella A. Climate change and mycotoxins—the African experience. Croat J Food Sci Technol. 2019;11(2):283–90. https://doi.org/10.17508/CJFST.2019.11.2.09.
Samson RA, Visagie CM, Houbraken J. Phylogeny, identification and nomenclature of the genus Aspergillus. Stud Mycol. 2014;78:141–217. https://doi.org/10.1016/j.simyco.2014.07.004.
Tao Y, Xie S, Xu F, Liu A, Chen D, Yuan Z. Ochratoxin A: toxicity, oxidative stress and metabolism. Food Chem Toxicol. 2018;112:320–31.
Omotayo OP, Omotayo AO, Mwanza M, Babalola O. Prevalence of mycotoxins and their consequences on human health. Toxicol Res. 2019;35(1):1–7. https://doi.org/10.5487/TR.2019.35.1.001.
Guan Y, Chen J, Nepovimova E, Long M, Wu W, Kuca K. Aflatoxin detoxification using microorganisms and enzymes. Toxins. 2021;13(1):46. https://doi.org/10.3390/toxins13010046.
EuropeanCommission. Commission REGULATION (EC) No. 1881/2006. Setting maximum levels for certain contaminants in foodstuffs. Off J Eur Un L. 2006;364:19.
Hahn M. The rising threat of fungicide resistance in plant pathogenic fungi: botrytis as a case study. J Chem Biol. 2014;7:133–41. https://doi.org/10.1007/s12154-014-0113-1.
Martínez-Culebras PV, Gandía M, Garrigues S, Marcos JF, Manzanares P. Antifungal peptides and proteins to control toxigenic fungi and mycotoxin biosynthesis. Int J Mol Sci. 2021;22:13261. https://doi.org/10.3390/ijms222413261.
Rtibi K, Selmi S, Grami D, Amri M, Eto B, El-Benna J, Sebai H, Marzouki L. Chemical constituents and pharmacological actions of carob pods and leaves (Ceratonia siliq ua L.) on the gastrointestinal tract: a review. Biomed Pharmacother. 2017;93:522–8. https://doi.org/10.1016/j.biopha.2017.06.08.
Rasheed DM, El-Kersh DM, Farag MA. Ceratonia siliqua (carob-locust bean) outgoing and potential trends of phytochemical, economic and medicinal merits. In: Mariod AA, editor. Wild fruits composition, nutritional value and products. Cham: Springer; 2019. p. 481–98.
Santonocito D, Granata G, Geraci C, Panico A, Siciliano EA, Raciti G, Puglia C. Carob seeds: Food waste or source of bioactive compounds. Pharmaceutics. 2020;12:1090. https://doi.org/10.3390/pharmaceutics12111090.
Fidan H, Stankov S, Petkova N, Petkova Z, Iliev A, Stoyanova M, Ivanova T, Zhelyazkov N, Ibrahim S, Stoyanova A. Evaluation of chemical composition, antioxidant potential and functional properties of carob (Ceratonia siliqua L.) seeds. J Food Sci Technol. 2020;57:2404–13. https://doi.org/10.1007/s13197-020-04274-z.
Ayaz FA, Torun H, Glew RH, Bak ZD, Chuang LT, Presley JM, Andrews R. Nutrient content of carob pod (Ceratonia siliqua L.) flour prepared commercially and domestically. Plant Foods Hum Nutr. 2009;64(4):286–92.
Karaca OB, Saydam IB, Güven M. Physicochemical, mineral and sensory properties of set-type yoghurts produced by addition of grape, mulberry and carob molasses (Pekmez) at different ratios. Int J Dairy Technol. 2011;65:111–7. https://doi.org/10.1111/j.1471-0307.2011.00731.x.
Loullis A, Pinakoulaki E. Carob as cocoa substitute: a review on composition, health benefits and food applications. Eur Food Res Technol. 2018;244:959–77. https://doi.org/10.1007/s00217-017-3018-8.
Goulas V, Georgiou E. Utilization of carob fruit as sources of phenolic compounds with antioxidant potential: extraction optimization and application in food models. Foods. 2020;9:20. https://doi.org/10.3390/foods9010020.
Prior RL, Wu X, Schaich K. Standard methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem. 2005;53(10):4290–302. https://doi.org/10.1021/jf0502698.
Yan-Hwa C, Chao-Lin C, Hsia-Fen H. Flavonoid content of several vegetables and their antioxidant activity. J Sci Food Agric. 2000;80:561–6.
Ayoola GA, Coker HA, Adesegun SA, Adepoju-Bello AA, Obaweya K, Ezennia EC, Atangbayila TO. Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in Southwestern Nigeria. Trop J Pharm Res. 2008;7:1019–24. https://doi.org/10.4314/tjpr.v7i3.14686.
Lee YH, Choo C, Watawana MI, Jayawardena N, Waisundara VY. An appraisal of eighteen commonly consumed edible plants as functional food based on their antioxidant and starch hydrolase inhibitory activities. J Sci Food Agric. 2015;95:2956–64. https://doi.org/10.1002/jsfa.703.
Julkunen-Titto R. Phenolic constituents in the leaves of northern willows: methods for the analysis of certain phenolics. J Agric Food Chem. 1985;33(2):213–7.
Perez C, Paul M, Bazerque P. Antibiotic assay by agar well diffusion method. Acta Biol Med Exp. 1990;15:113–5.
El-Desouky TA. Evaluation of effectiveness aqueous extract for some leaves of wild edible plants in Egypt as anti-fungal and anti-toxigenic. Heliyo. 2021;7(2):e06209. https://doi.org/10.1016/j.heliyon.2021.e06209.
Ammar HAM, El-Desouky TA. Green synthesis of nanosilver particles by Aspergillus terreus HA1N and Penicillium expansum HA2N and its antifungal activity against mycotoxigenic fungi. J Appl Microbiol. 2016;121:89–100. https://doi.org/10.1111/jam.13140.
AOAC. Association of official analytical chemists official methods of analysis of AOAC international 17th ed., nature toxins. Arlington: AOAC International; 2007.
El-Desouky T, Hussain Hassan B H. Influence of natural capsaicin on aflatoxins produced by Aspergillus aflatoxiformans in grains. Arc Phytopathol Plant Prot. 2021. https://doi.org/10.1080/03235408.2021.1999749.
Nawel O. Phytochemical analysis and antioxidant activity of the flavonoids extracts from pods of Ceratonia siliqua L. J Pharm Pharm. 2017;4:59–165. https://doi.org/10.15436/2377-1313.17.1490.
Ayache SB, Saafi EB, Emhemmed F, Flamini G, Achour L, Muller CD. Biological activities of aqueous extracts from Carob. Plant (Ceratonia siliqua L.) by antioxidant. Analg Proapoptotic Prop Eval Mol. 2020;25:1–16. https://doi.org/10.3390/molecules25143120.
Turhan I. Relationship between sugar profile and D-Pinitol content of pods of wild and cultivated types of Carob Bean (Ceratonia siliqua L.). Int J Food Prop. 2014;17:363–70. https://doi.org/10.1080/10942912.2011.631255.
El Bouzdoudi B, El Ansari ZN, Mangalagiu I, Mantu D, Badoc A, Lamarti A. Determination of polyphenols content in carob pulp from wild and domesticated Moroccan trees. Am J Plant Sci. 2016;7:1937–51. https://doi.org/10.4236/ajps.2016.714177.
Mellado-Mojica E, Seeram NP, López MG. Comparative analysis of maple syrups and natural sweeteners: carbohydrates composition and classification (differentiation) by HPAEC-PAD and FTIR spectroscopy-chemometrics. J Food Compos Anal. 2016;52:1–8. https://doi.org/10.1016/j.jfca.2016.07.001.
Christou C, Agapiou A, Kokkinofta R. Use of FTIR spectroscopy and chemometrics for the classification of carobs origin. J Adv Res. 2018;10:1–8. https://doi.org/10.1016/j.jare.2017.12.001.
Papagiannopoulos M, Wollseifen HR, Mellenthin A, Haber B, Galensa R. Identification and quantification of polyphenols in carob fruits (Ceratonia siliqua L.) and derived products by HPLC-UV-ESI/MSN. J Agric Food Chem. 2004;52:3784–91. https://doi.org/10.1021/jf030660y.
Cavdarova M, Makris DP. Extraction kinetics of phenolics from carob (Ceratonia siliqua L.) kibbles using environmentally benign solvents. Waste Biomass Valoriz. 2014;5:773–9. https://doi.org/10.1007/s12649-014-9298-3.
Tounsi L, Ghazala I, Kechaou N. Physicochemical and phytochemical properties of Tunisian carob molasses. Food Meas. 2020;14:20–30. https://doi.org/10.1007/s11694-019-00263-9.
Darwish WS, Khadr AES, Kamel M, Abd Eldaim MA, El Sayed IET, Abdel-Bary HM, Ullah S, Ghareeb DA. Phytochemical characterization and evaluation of biological activities of Egyptian carob pods (Ceratonia siliqua L.) aqueous extract in vitro study. Plants. 2021;10:2626. https://doi.org/10.3390/plants10122626.
Lakkab I, El Hajaji H, Lachkar N, Lefter R, Ciobica A, El Bali B, Lachkar M. Ceratonia siliqua L. seed peels: phytochemical profile, antioxidant activity, and effect on mood disorders. J Funct Food. 2019;54:457–65. https://doi.org/10.1016/j.jff.2019.01.041.
Hao B, Cheng S, Clancy CJ, Nguyen MH. Caspofungin kills Candida albicans by causing both cellular apoptosis and necrosis. Antimicrob Agents Chemother. 2013;57:326–32. https://doi.org/10.1128/AAC.01366-12.
Da X, Nishiyama Y, Tie D, Hein KZ, Yamamoto O, Morita E. Antifungal activity and mechanism of action of Ou-gon (Scutellaria root extract) components against pathogenic fungi. Sci Rep. 2019;9(1):1683. https://doi.org/10.1038/s41598-019-38916.
Loi M, Paciolla C, Logrieco A, Mule G. Plant bioactive compounds in pre- and post-harvest management for aflatoxins reduction. Front Microbiol. 2020. https://doi.org/10.3389/fmicb.2020.00243.
Karapynar M. Inhibition effects of some spice agents on aflatoxigenic mould growths. Proceedings of the international food symposium. Bursa, Turkey. 4–6 Apr1989; pp: 129–137.
Satish S, Mohana DC, Ranhavendr MP, Raveesha K. Antifungal activity of some plant extracts against important seed borne pathogens of Aspergillus sp. J Agric Technol. 2007;3:109–19.
Pundir RK, Jain P. Antifungal activity of twenty-two ethanolic plant extracts against food-associated fungi. J Pharmacy Res. 2010;3:506–10.
Pereira MC, Chalfoun SM, Pimenta CJ, Angelico CL, Maciel WP. Spices, fungi mycelial development and ochratoxin A production. Sci Res Essay. 2006;1:38–42.
Ren Y, Jin J, Zheng M, Yang Q, Xing F. Ethanol inhibits Aflatoxin B1 biosynthesis in Aspergillus flavus by up-regulating oxidative stress-related genes. Front Microbiol. 2020;10:2946. https://doi.org/10.3389/fmicb.2019.02946.
Buitimea-Cantua GV, Buitimea-Cantua NE, Rocha-Pizana MDR, Hernandez-Morales A, Magana-Barajas E, Molina-Torres J. Inhibitory effect of Capsicum chinense and Piper nigrum fruits, capsaicin and piperine on aflatoxins production in Aspergillus parasiticus by downregulating the expression of aflD, aflM, aflR, and aflS genes of aflatoxins biosynthetic pathway. J Environ Sci Health B. 2020;55(9):835–43. https://doi.org/10.1080/03601234.2020.1787758.
Mahmoud A. Inhibition of growth and aflatoxin biosynthesis of Aspergillus flavus by extracts of some Egyptian plants. L Appl Microbiol. 1999;29:334–6. https://doi.org/10.1046/j.1472-765x.1999.00636.
Passone MA, Resnik S, Etcheverry MG. The potential of food grade antioxidants in the control of Aspergillus section Flavi, interrelated mycoflora and aflatoxin B1 accumulation on peanut grains. Food Control. 2008;19:364–71. https://doi.org/10.1016/j.foodcont.2007.04.014.
Passone MA, Girardi NS, Etcheverry M. Antifungal and anti-aflatoxigenic activity by vapor contact of three essential oils, and effects of environmental factors on their efficacy. Food Sci Technol. 2013;53:434–44. https://doi.org/10.1016/j.lwt.2013.03.012.
Romero SM, Alberto MR, Vaamonde G. Effect of gallic acid on Aspergillus carbonarius growth and ochratoxin A production. World Mycotox J. 2010;3:45–8. https://doi.org/10.3920/WMJ2009.1181.
Palumbo J, O’Keeffe T, Mahoney N. Inhibition of ochratoxin A production and growth of Aspergillus species by phenolic antioxidant compounds. Mycopathologia. 2007;164:241–8. https://doi.org/10.1007/s11046-007-9057-0.
Bisogno F, Mascoti L, Sanchez C, Garibotto F, Giannini F, Kurina-Sanz M, Enriz R. Structure-antifungal activity relationship of cinnamic acid derivatives. J Agric Food Chem. 2007;55:10635–40. https://doi.org/10.1021/jf0729098.
Author information
Authors and Affiliations
Contributions
Tarek A. El-Desouky devised and designed the experiments. performed the experiments, and also analysed and interpreted the data; wrote the paper. The author read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
There are no competing interests to declare for this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Desouky, T.A. Protect peanut kernels from Aspergillus spp and their mycotoxins during storage by aqueous extract of carob pulp. Discov Food 2, 25 (2022). https://doi.org/10.1007/s44187-022-00026-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44187-022-00026-4