Abstract
Background
High-dose cytarabine (HDAC) is commonly used for consolidation therapy in young acute myeloid leukemia (AML) patients, but the dosage of cytarabine is still controversial in the clinic due to its obvious post-chemotherapy adverse effects. The aim of this study was to contrast the efficacy in different dose groups of cytarabine after consolidation therapy in Chinese AML patients.
Methods
AML patients treated with cytarabine consolidation at Qilu Hospital, Shandong University from January 2010 to September 2022 were retrospectively analyzed, from which 346 AML patients with relatively complete follow-up data were selected for this study. We compared the patients’ overall survival (OS) rate, relapse-free survival (RFS) rate, and hematologic adverse events in terms of their general characteristics, cytarabine consolidation therapy dose, consolidation course, 2022 European Leukemia Net (ELN) risk stratification, and transplantation.
Results
In AML patients under 60 years of age, the 5-year RFS rate with high-dose cytarabine consolidation therapy was superior to that of small-dose cytarabine (P = 0.024), while the 5-year RFS rate was comparable in the high-dose and intermediate-dose groups, and there was no obvious difference among the three groups in the 5-year OS rate (P > 0.05). OS and RFS of those given more than 3 courses of cytarabine consolidation therapy were better than those in the 1–2 courses group (P = 0.060, P = 0.040). OS and RFS were better in patients with cumulative dose of cytarabine ≥ 36g than in patients with cumulative dose < 36g (P < 0.05), but cumulative dose ≥ 54g was comparable in OS and RFS with ≥ 36–< 54g group (P > 0.05). There was no significant difference in hematologic adverse effects among the three treatment groups.
In the latest ELN risk stratification favorable-risk group, the cumulative dose of cytarabine ≥ 36g had a better 5-year RFS rate than the < 36g group (P = 0.038), and in the intermediate-risk group the 5-year OS rate and RFS rate were better in the ≥ 36g group than the < 36g group (P = 0.012, 0.025). In addition, the prognosis of transplanted patients was better than that of non-transplanted patients, whereas in non-transplanted patients, consolidation therapy with ≥ 36g cytarabine can effectively improve outcomes. Multivariate analysis indicated that age, fibrinogen (FIB) and the cumulative dose of cytarabine of ≥ 36–< 54g were predictors of OS, while age, white blood cell (WBC) and HDAC were predictors of RFS.
Conclusion
The results of the study showed that consolidation therapy with cytarabine up to a cumulative dose of ≥ 36–< 54g in AML patients who did not undergo transplantation significantly improved patient prognosis. In the latest ELN risk stratification, cumulative doses of cytarabine ≥ 36g had a better prognosis in favorable and intermediate-risk patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Acute myeloid leukemia (AML) is a malignant tumor caused by an abnormal expansion of large numbers of marrow blasts leading to suppression of bone marrow hematopoiesis [1]. Treatment includes induction therapy and consolidation therapy after remission. The most common induction therapy is “3 + 7” regimen based on anthracyclines combined with cytarabine, followed by consolidation therapy to minimize residual and prevent recurrence [2]. Consolidation regimens contain high-dose cytarabine chemotherapy, combination therapy with cytarabine and anthracyclines, as well as hematopoietic stem cell transplantation (HSCT) [3].
Studies have shown that in young adult patients, high-dose cytarabine results in longer survival than multi-agent sequential chemotherapy in intermediate- and high-risk patients [4, 5]. Furthermore, patients consolidated on high-dose cytarabine alone in younger patients have a better prognosis than those on small dose [6, 7]. However, there was no difference in outcome between high-dose cytarabine and intermediate-dose cytarabine consolidation therapy [8]. Taking into account the toxicity of high-dose cytarabine and its impact on prognosis, the 2022 ELN guidelines suggest that the consolidation therapy with high-dose cytarabine is not recommended, and that similar efficacy can be achieved with intermediate-dose cytarabine [9].
To further clarify the optimal dose of cytarabine in AML patients on consolidation therapy and its significance in the latest ELN risk stratification, we retrospectively analyzed the clinical characteristics of newly diagnosed AML patients under the age of 60 years in our hospital from January 2010 to September 2022, and comparatively analyzed the prognosis and adverse reactions of consolidation therapy with cytarabine alone at different dose, courses of treatment, cumulative dose, and cytogenetic risks, in order to supply more reliable abstract basis for the consolidation therapy of AML in China.
2 Methods
2.1 Patients
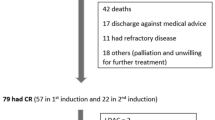
This study retrospectively collected AML patients from January 2010 to September 2022 at Qilu Hospital, Shandong University who met the following criteria: 1) 14–59 years old; 2) Eastern Cooperative Oncology Group (ECOG) score ≤ 2; 3) reached complete remission (CR) after 1 or 2 courses of induction therapy and consolidated with 1 or more cytarabine regimens. Patients with acute promyelocytic leukemia, AML transformed by myelodysplastic syndromes, and patients with combined cardiac, hepatic, renal, and other important organ insufficiency were excluded (Fig. 1). The study was approved by the Ethics Committee of Qilu Hospital, Shandong University, and acted in accordance with the Declaration of Helsinki.
2.2 Treatment
Induction therapy: including standard “3 + 7” combination regimens such as idarubicin combined with cytarabine (IA), daunorubicin combined with cytarabine (DA), homoharringtonine combined with cytarabine (HA), with or without molecularly targeted drugs (including venetoclax, Sorafenib, etc.). For patients who cannot tolerate the “3 + 7” regimen, the less toxic granulocyte colony-stimulating factor combined with cytarabine and aclarubicin (CAG) regimen is mostly used for induction. Efficacy assessment of the therapy was carried out after the 1st course, and if CR was not achieved, the 2nd course of induction therapy was continued. Patients were excluded if CR was still not achieved after the 2nd course of treatment. Patients who achieved CR on induction therapy were further determined by multiparametric flow cytometry to detect their minimal residual disease (MRD).
Consolidation therapy: after CR, individualized consolidation therapy was used according to the patient’s own condition. According to the single-dose of cytarabine in each consolidation therapy, patients were divided into 3 groups: high-dose cytarabine (HDAC) regimen (2.0 g/m2-3.0 g/m2, q12h, day 1–3), intermediate-dose cytarabine (IDAC) regimen (1.0 g/m2-1.9 g/m2, q12h, day 1–3) and small-dose cytarabine (SDAC) regimen (0.5 g/m2-0.9 g/m2, q12h, day 1–3), in some cases alternating with the standard “3 + 7” regimen of consolidation chemotherapy. The cumulative dose of cytarabine was defined as: the single dose in each therapy × the body surface area × 2 × the number of days in each therapy × the cycles of therapy. Based on above, patients were categorized into high cumulative dose group (≥ 54g), medium cumulative dose group (≥ 36–< 54g) and low cumulative dose group (< 36g). Patients using different cycles were also divided into the 1–2 courses group and the ≥ 3 courses group.
2.3 Assessment criteria
Overall survival (OS): the time from the patient’s diagnosis until the patient’s death from any cause, loss to follow-up, or the end of follow-up. Relapse-free survival (RFS): the time from the patient’s first complete remission to relapse, death, loss to follow-up, or end of follow-up. Complete remission (CR): bone marrow primitive cells < 5%; peripheral blood neutrophil count ≥ 1 × 109/L; platelet count ≥ 100 × 109/L; no extramedullary disease; normal cytogenetics. Relapse: marrow blasts ≥ 5%, or leukemia cells found in peripheral blood or cerebrospinal fluid [9].
2.4 Statistical analysis
Patient information was statistically analyzed using SPSS 25.0. The general characteristics of the patients were analyzed by chi-square tests or non-parametric tests. Survival curves were analyzed using Kaplan-Meier, comparisons between groups were made using Log-rank test, and hazard ratios (HR) and 95% confidence intervals (CI) were estimated using Cox model. Univariate and multivariate Cox regression analyses were performed to determine independent predictors of OS and RFS. P values less than 0.05 were considered significant. Variables with P values less than 0.2 in the univariate analysis were included in the backward method multivariate analysis. A two-sided P < 0.05 indicated statistical significance.
3 Results
3.1 Clinical characteristics of patients in different single-dose groups
Three hundred forty-six patients with CR were evaluated. Of these, 186 patients were treated with HDAC, 70 patients with IDAC, and 90 patients with SDAC. Comparison of clinical characteristics such as age, gender, 2022 ELN risk stratification, WBC, hemoglobin (HGB), and platelet (PLT) counts at the time of first diagnosis among the three groups of patients, showed significant differences in risk stratification, different molecular types, MRD, transplantation, and relapse(P < 0.05) (Table 1). 346 patients had an overall median follow-up time of 26 (2–160) months, with a median follow-up time of 26 (9–160) months for survivors months, and 77 patients (22.3%) were lost to follow-up. A total of 71 patients (20.5%) received HSCT. A total of 112 patients (32.4%) relapsed during the follow-up period.
3.2 Efficacy in different single-dose groups of cytarabine
When patients were grouped according to the single-dose of cytarabine administered, the 5-year OS rate was 52.7% in the HDAC group, 48.6% in the IDAC group, and 37.8% in the SDAC group, while the differences among the three groups were not statistically significant (P = 0.781, 0.199, 0.070). The median OS was not reached in the HDAC group, whereas the median OS was 38.03 (95% CI: 21.47-54.59) months and 28.93 (95% CI: 13.77-44.09) months in the IDAC and SDAC groups (Fig. 2a). The 5-year RFS rates were 47.8% in the HDAC group, 42.9% in the IDAC group, and 30.0% in the SDAC group (P = 0.585, 0.227, 0.024). The median RFS in the three groups was 35.67 (95% CI: 24.71–46.63), 19.03 (95% CI: 4.21–33.85), and 17.77 (95% CI: 13.15–22.39) months, respectively (Fig. 2b).
OS and RFS in different groups of AML patients. a Comparison of OS among the HDAC, IDAC and SDAC groups; b Comparison of RFS among the HDAC, IDAC and SDAC groups; c Comparison of OS between the 1–2 courses group and the ≥ 3 courses group; d Comparison of RFS between the 1–2 courses group and the ≥ 3 courses group; e Comparison of OS among the ≥ 54 g, ≥ 36–< 54g, and < 36 g groups; f Comparison of RFS among the ≥ 54g, ≥ 36–< 54g, and < 36g groups
3.3 Efficacy in different course groups of cytarabine
Grouped according to the cycle of cytarabine consolidation therapy, there were 220 patients in the 1–2 courses group and 126 patients in the ≥ 3 courses group. The 5-year OS rate was 51.6% in the ≥ 3 courses group and 45.9% in the 1–2 courses group, and the difference was slightly there (P = 0.06). The median OS was 30.27 (95% CI: 14.13–46.41) months in the 1–2 courses group and 42.63 (95% CI: 29.02–56.24) months in the ≥ 3 courses group (Fig. 2c). The 5-year RFS rate was 44.4% in the ≥ 3 courses group, superior to the rate of 40.9% in the 1–2 courses group (P = 0.04). The median RFS was 17.77 (95% CI: 12.63–22.91) months in the 1–2 courses group and 33.63 (95% CI:21.19–43.07) months in the ≥ 3 courses group (Fig. 2d).
3.4 Efficacy in different cumulative dose groups of cytarabine
Grouped by different cumulative dose, there were 102 patients in the high cumulative dose group, 73 patients in the medium cumulative dose group, and 171 patients in the low cumulative dose group. The 5-year OS rates in the three groups were 52.0%, 63.0%, and 39.2%, respectively; the 5-year OS rate of the high cumulative dose group and the medium cumulative dose group were better than those of the low cumulative dose group (P = 0.021, 0.001), but there was no significant difference between the high cumulative dose group and the medium cumulative dose group (P = 0.217). The median OS was not achieved in the high cumulative dose group or the medium cumulative dose group, and the median OS of the low cumulative dose group was 23.47 (95% CI: 12.77–34.18) months (Fig. 2e). The 5-year RFS rates in the three groups were 48.0%, 54.8%, and 33.3%. The 5-year RFS rates of the high cumulative dose group and the medium cumulative dose group were better than those of the low cumulative dose group (P = 0.003, 0.008), but the efficacy of the high cumulative dose was not significantly different from that of the medium cumulative dose (P = 0.757). The median RFS of the high cumulative dose group was 36.80 (95% CI: 26.60–47.00) months, while 17.03 (95% CI: 13.58–20.49) months in the low cumulative dose group (Fig. 2f), however not reached in the medium cumulative dose group.
3.5 Efficacy in different risk stratification groups
Patients were grouped according to different risk stratification, and the number of patients in the favorable, intermediate and adverse risk groups were 167, 166 and 13. In the favorable-risk group, 107 and 60 patients were divided with cumulative cytarabine dose ≥ 36g and < 36g. The 5-year OS rate in the cumulative dose ≥ 36g group was 57.0%, better than 45.0% in the < 36g group, but the difference was not statistically significant (P = 0.089). The median OS was not reached in the ≥ 36g group, while in the < 36g group was 33.87 (95% CI: 10.92–56.82) months (Fig. 3a). The 5-year RFS rate in the ≥ 36g group was 52.3% superior to 38.3% in the < 36g group (P = 0.038); median RFS was not reached in the ≥ 36g group, and 20.53 (95% CI: 13.11–27.95) months in the < 36g group (Fig. 3b). In the intermediate-risk group, 61 and 105 patients were divided with cumulative cytarabine dose: ≥ 36g group versus the < 36g group. The 5-year OS rate in the ≥ 36g group was 54.1% superior to that in the < 36g group, which was 36.2% (P = 0.012); the median OS was not reached in the ≥ 36g group, and the median OS in the < 36g group was 22.47 (95% CI: 9.34–35.60) months (Fig. 3c). The 5-year RFS rate in the ≥ 36g group was 45.9% superior to 30.5% in the < 36g group (P = 0.025); the median RFS was 39.43 (95% CI: 17.13–61.73) months in the ≥ 36g group and 15.43 (95% CI: 10.77–20.09) months in the < 36g group (Fig. 3d). In the high-risk group, there were 7 and 6 patients with cumulative cytarabine dose ≥ 36g and < 36g. The 5-year OS rate in the cumulative dose ≥ 36g group was 71.4% better than 33.3% in the < 36g group (P = 0.443), while the median OS was not reached in the ≥ 36g group and was 14.30 (95% CI: 3.29–25.31) months in the < 36g group (Fig. 3e). The 5-year RFS rate in the ≥ 36g group was 71.4% superior to 33.3% in the < 36g group (P = 0.443), the median RFS was not reached in the ≥ 36g group, and the median RFS was 14.30 (95% CI: 3.86–24.74) months in the < 36 g group (Fig. 3f).
OS and RFS of AML patients according to ELN risk stratification. a Probability of OS for favorable-risk patients; b Probability of RFS for favorable-risk patients; c Probability of OS for intermediate-risk patients; d Probability of RFS for intermediate-risk patients; e Probability of OS for adverse-risk patients; f Probability of RFS for adverse-risk patients
3.6 Efficacy in patients with different somatic mutation features
Among AML patients grouped according to cytogenetics or gene rearrangements, there were 72 AML1-ETO-positive patients, 26 CBFB-MYH11-positive patients, 37 NPM1-mutated patients, 37 CEBPA-mutated patients (24 CEBPA bZIP mutated patients) and 26 FLT3-ITD-positive patients. Among AML1-ETO-positive patients, 5-year OS (81.8% vs. 31.3%, P = 0.016) and RFS (72.7% vs. 39.3%, P = 0.049) were better in patients consolidated with medium cumulative dose of cytarabine than in those with low cumulative dose, however the difference between the high cumulative dose and the medium cumulative dose was not statistically significant (P = 0.074,0.179). In other cytogenetics or gene rearrangements, there was no significant difference in prognosis by cumulative dose (P > 0.05).
3.7 Impact of transplantation
HSCT was performed in 37 (21.1%) and 34 (19.9%) patients in the cumulative dose ≥ 36g and < 36g groups. The 5-year OS rate reached 85.9% in transplanted patients, whereas only 38.2% in patients without transplantation (P < 0.01). The median OS was not reached in transplanted patients, while 35.70 (95% CI: 25.22–46.18) months in patients without transplantation (Fig. 4a). The 5-year RFS rate was 80.3% in transplanted patients, which was higher than that of 32.4% in patients without transplantation (P < 0.01). The median RFS was not reached in transplanted patients, and 17.50 (95% CI: 14.69–20.31) months in patients without transplantation (Fig. 4b). In transplanted patients, the 5-year OS rates were 89.2% and 82.4% (P = 0.402) in the cumulative dose ≥ 36g and < 36g group, and the median OS was not reached (Fig. 4c). The 5-year RFS rates were 83.8% and 76.5% (P = 0.400) in the cumulative dose ≥ 36g and < 36g groups, and the median RFS was not reached in any of them (Fig. 4d). Among patients who did not undergo transplantation, the 5-year OS rate in the ≥ 36g group was 47.8%, which was better than 28.5% in the < 36g group (P < 0.01), and the 5-year median OS was 35.67 (95% CI: 18.82–52.53) and 17.40 (95% CI: 11.74–23.06) months (Fig. 4e). The 5-year RFS rate in the ≥ 36g group was 42.0%, which was higher than that of the < 36g group which was 22.6% (P < 0.01); the 5-year median RFS was 26.30 (95% CI: 13.60–39.00) months and 14.20 (95% CI:11.49–16.91) months (Fig. 4f).
Effect of transplantation on the survival of AML patients with cytarabine consolidation. a The 5-year OS rate of AML patients with or without transplantation; b The 5-year RFS rate of AML patients with or without transplantation; c For the transplantation patients, the 5-year OS rate in the cumulative dose ≥ 36g and < 36g groups; d For the transplantation patients, the 5-year RFS rate in the cumulative dose ≥ 36g and < 36g groups; e For patients without transplantation, the 5-year OS rate in the cumulative dose ≥ 36g and < 36g groups; f For patients without transplantation, the 5-year RFS rate in the cumulative dose ≥ 36g and < 36g groups
3.8 Multifactorial COX regression analysis of RFS among AML patients
In the univariate analysis of OS, we analyzed age, ELN risk stratification, WBC, HGB, PLT, FIB, LDH, single dose of cytarabine, consolidation cycle, and cumulative dose of cytarabine. Univariate analysis showed a significant association between OS and cumulative dose. Variables with P value less than 0.2 in the univariate analysis, including age, single dose of cytarabine, consolidation cycle, cumulative dose of cytarabine, WBC, PLT and FIB were included in the multivariate analysis. Multivariate analysis showed that age (P = 0.029), FIB (P = 0.047) and cumulative dose (≥ 36–< 54g vs. < 36g) (P = 0.016) were associated with OS (Table 2). Univariate analysis in RFS showed that WBC, single dose of cytarabine, consolidation cycle, and cumulative dose of cytarabine were significantly associated with RFS. Age (P = 0.027), WBC (P = 0.014), and single dose (HDAC vs. SDAC) (P = 0.001) were associated with RFS based on multivariate regression analysis (Table 3).
3.9 Hematological adverse reactions
Hematological adverse reactions, including neutropenia, anemia and thrombocytopenia, were also collected among patients in different cumulative dose groups of cytarabine in this study. It was found that the rate of hematologic adverse reactions was higher in the high cumulative dose group, except for some patients who were not reviewed, but no statistically significant differences were seen (Table 4). The duration of neutrophil deficiency was 5 (IQR: 3–17) days in the high cumulative dose group, 5.5 (IQR: 3–12) days in the medium cumulative dose group, and 4 (IQR: 3–10) days in the low cumulative dose group (P = 0.175).
4 Discussion
In order to prevent relapse after CR of AML, it is important to provide further intensive treatment for AML patients, which can control the risk of disease relapse by removing the residual leukemia cells and reducing the body’s resistance to drugs, thus effectively prolonging the survival of patients [10]. Since the Cancer and Leukemia Group B-8525 trial (CALGB-8525) in 1994, by randomizing different cytarabine doses (100 mg/m2,400 mg/m2,3 g/m2) into a controlled trial, it was found that high-dose cytarabine was more advantageous than small-dose cytarabine treatment in AML patients under 60 years old [6]. However, this trial did not show the duration, cumulative dose, or toxic effects of cytarabine consolidation therapy. In a subsequent study, it was shown that high-dose cytarabine and intermediate-dose cytarabine consolidation therapy had comparable prognosis for patients, with no statistically significant difference [11, 12]. Compared to small-dose cytarabine, high-dose cytarabine has a better survival rate for patients [13]. Survival was also significantly improved with intermediate-dose cytarabine compared with small-dose cytarabine and both had similar adverse effects after treatment [14]. For the cycle of cytarabine use study, 4 courses of treatment were found to improve over 3 courses of treatment in terms of cumulative incidence rate (CIR) and RFS [15]. As for the frequency of cytarabine use, the study showed that administration every 12 h on days 1, 2 and 3 was more conducive to promoting recovery from hematologic adverse effects and shortening the length of hospitalization [16].
In patients with different cytogenetic risk, HDAC consolidation therapy is more effective than IDAC or standard-dose cytarabine-based multiagent sequential treatment cycle [17, 18]. However, recent studies have shown that there is no significant difference in OS and RFS between IDAC and HDAC in intermediate- and adverse-risk patients, and that HDAC may increase the risk of septic shock in patients [19]. Therefore, in order to provide more real-world data about the optimal cytarabine dose level, OS and RFS were chosen as the primary endpoints of this study in patients with AML treated with consolidation of cytarabine alone in our institution after January 2010, and the authors aimed to find the appropriate consolidation dose of cytarabine as early as possible.
In this retrospective study, compared with SDAC, we found that HDAC consolidation therapy had a significant advantage in RFS in AML patients younger than 60 years of age, with no significant difference in OS and comparable efficacy of HDAC to IDAC. In addition, comparing the courses of cytarabine consolidation therapy yielded significant differences in OS and RFS between the ≥ 3 courses group and the 1–2 courses group, which is the same as the results of previous studies [20].
In response to the different individual differences and tolerance of Chinese patients, the single dose and duration of treatment using cytarabine were also different, so we grouped them by cumulative dose in order to further determine the prognostic impact. It was shown that patients with total cumulative dose of 36g or more had longer OS and RFS, but there was no significant difference in prognosis between patients with ≥ 54g and those with ≥ 36–< 54g. In the latest 2022 ELN risk stratification, cumulative dose of cytarabine ≥ 36g had better survival in favorable and intermediate-risk patients, whereas there was no significant difference in adverse-risk patients, which may be related to the fact that fewer adverse-risk patients were consolidated with cytarabine. Meanwhile, we compared the prognostic impact of different cumulative dose of cytarabine consolidation therapy on patients in 2022 ELN and 2017 ELN risk stratification, respectively [9, 21], the results showed comparable prognostic impacts of the two stratification methods on patients. In further investigating the effect of cytogenetics or gene rearrangements on patients, we found that AML1-ETO-positive patients were more sensitive to cytarabine in the medium cumulative dose group than in the low cumulative dose group, but there was no difference between the high cumulative dose and the medium cumulative dose. The effect of cytarabine was not found in other genes, which may be related to the small sample size.
In addition to cytarabine consolidation therapy, HSCT as the most effective post-remission therapy, is not available to all patients due to the high rate of complications associated with its treatment as well as the greater difficulty in finding a suitable donor [22, 23]. For patients receiving transplantation, the 5-year OS and RFS in the cumulative dose ≥ 36g group of cytarabine were similar to those in the cumulative dose < 36g group, suggesting that for patients receiving transplantation, there was no survival difference between receiving the cumulative dose of cytarabine ≥ 36g or < 36g regimen prior to transplantation. However, for patients not undergoing transplantation, the 5-year OS and RFS were obviously higher in the cumulative dose ≥ 36g group than in the cumulative dose < 36g group, suggesting that cumulative dose of cytarabine ≥ 36g group consolidation is the preferred regimen for patients with AML who do not have the opportunity to undergo transplantation. With the increasing research into the molecular and genetic mechanisms of AML pathogenesis, more and more targeted drugs are entering the clinic to prolong patients’ survival [24, 25]. We explored whether the application of targeted drugs had an impact on the conclusions of our study. In the total population, Sorafenib was added to the consolidation therapy in 10 patients, of which 2 patients have already combined Sorafenib to induction therapy. 7 patients were treated with venetoclax in the induction therapy, and 2 used venetoclax for consolidation. There was no significant difference in OS and RFS between patients using Sorafenib or venetoclax compared with patients not using targeted drugs (P = 0.363, 0.427). Considering that there were fewer patients using targeted drugs, their impact on the results of this study were not statistically significant.
In conclusion, the present study suggests that consolidation with cytarabine may be preferred in young adult AML patients in the absence of a suitable donor. HDAC consolidation yields higher relapse-free survival than SDAC, especially with cumulative dose of ≥ 36–< 54g having longer survival in favorable and intermediate-risk patients. Our study has some shortcomings, such as a single-center retrospective study with an insufficient sample size, data from medical record retrieval inevitably suffers from information bias, and a lack of treatment-related toxicities other than hematology. These questions can be confirmed by subsequent refinement of the follow-up content and multicenter large sample studies to draw more meaningful conclusions.
Availability of data and materials
Due to patient privacy this raw data is not publicly available and can be obtained by contacting the corresponding author upon reasonable request.
Abbreviations
- AML:
-
Acute myeloid leukemia
- OS:
-
Overall survival
- RFS:
-
Relapse-free survival
- ELN:
-
European Leukemia Net
- FIB:
-
Fibrinogen
- CR:
-
Complete remission
- HSCT:
-
Hematopoietic stem cell transplantation
- ECOG:
-
Eastern Cooperative Oncology Group
- IA:
-
Idarubicin combined with cytarabine
- DA:
-
Daunorubicin combined with cytarabine
- HA:
-
Homoharringtonine combined with cytarabine
- CAG:
-
Granulocyte colony-stimulating factor combined with cytarabine and aclarubicin
- HDAC:
-
High-dose cytarabine
- IDAC:
-
Intermediate-dose cytarabine
- SDAC:
-
Small-dose cytarabine
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- MRD:
-
Minimal residual disease
- WBC:
-
White blood cell
- HGB:
-
Hemoglobin
- PLT:
-
Platelet
- LDH:
-
Lactate dehydrogenase
- IQR:
-
Interquartile range
- CALGB-8525:
-
Cancer and Leukemia Group B-8525 trial
- CIR:
-
Cumulative incidence rate
References
Shallis RM, et al. Epidemiology of acute myeloid leukemia: recent progress and enduring challenges. Blood Rev. 2019;36:70–87.
DiNardo CD, et al. Acute myeloid leukaemia. Lancet. 2023;401(10393):2073–86.
Pollyea DA, et al. NCCN guidelines insights: acute myeloid leukemia, version 2.2021. J Natl Compr Canc Netw. 2021;19(1):16–27.
Thomas X, et al. Comparison of high-dose cytarabine and timed-sequential chemotherapy as consolidation for younger adults with AML in first remission: the ALFA-9802 study. Blood. 2011;118(7):1754–62.
Wang X, et al. High-dose cytarabine monotherapy is superior to standard-dose cytarabine- based multiagent sequential treatment cycle for consolidation treatment in adult (14–59 years) AML patients according to European Leukemia Net 2022 risk stratification. Front Oncol. 2022;12:1070588.
Mayer RJ, et al. Intensive postremission chemotherapy in adults with acute myeloid leukemia. Cancer and Leukemia Group B. N Engl J Med. 1994;331(14):896–903.
Garcia-Manero G, et al. A randomized phase III study of standard versus high-dose cytarabine with or without vorinostat for AML. Leukemia. 2024;38(1):58–66.
Hanoun M, et al. Intensified cytarabine dose during consolidation in adult AML patients under 65 years is not associated with survival benefit: real-world data from the German SAL-AML registry. J Cancer Res Clin Oncol. 2023;149(8):4611–21.
Döhner H, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140(12):1345–77.
Jimenez-Chillon C, Dillon R, Russell N. Optimal post-remission consolidation therapy in patients with AML. Acta Haematol. 2024;147(2):147–58.
Burnett AK, et al. Optimization of chemotherapy for younger patients with acute myeloid leukemia: results of the medical research council AML15 trial. J Clin Oncol. 2013;31(27):3360–8.
Tangchitpianvit K, et al. Efficacy and safety of consolidation therapy with intermediate and high dose cytarabine in acute myeloid leukemia patients. Hematology. 2021;26(1):355–64.
Weick JK, et al. A randomized investigation of high-dose versus standard-dose cytosine arabinoside with daunorubicin in patients with previously untreated acute myeloid leukemia a Southwest Oncology Group study. Blood. 1996;88(8):2841–51.
Ye L, et al. Intermediate dose cytarabine improves survival and relapse-free rate compared with standard-dose cytarabine as post-remission treatment for acute myeloid leukemia: a retrospection study. Medicine (Baltimore). 2021;100(3):e24273.
Burnett AK, et al. Defining the optimal total number of chemotherapy courses in younger patients with acute myeloid leukemia: a comparison of three versus four courses. J Clin Oncol. 2021;39(8):890–901.
Dumas PY, et al. Delivering HDAC over 3 or 5 days as consolidation in AML impacts health care resource consumption but not outcome. Blood Adv. 2020;4(16):3840–9.
Gong D, et al. Comparison of clinical efficacy of cytarabine with different regimens in postremission consolidation therapy for adult t(8;21) AML patients: a multicenter retrospective study in China. Acta Haematol. 2016;136(4):201–9.
Kolla BC, et al. High risk of relapse with intermediate dose cytarabine for consolidation in young favourable-risk acute myeloid leukaemia patients following induction with 7+3: a retrospective multicentre analysis and critical review of the literature. Br J Haematol. 2021;194(1):140–4.
Chanswangphuwana C, et al. Comparison of three doses of cytarabine consolidation for intermediate- and adverse-risk acute myeloid leukemia: real world evidence from Thai acute myeloid leukemia registry. Clin Lymphoma Myeloma Leuk. 2022;22(10):e915–21.
Byrd JC, et al. Repetitive cycles of high-dose cytarabine benefit patients with acute myeloid leukemia and inv(16)(p13q22) or t(16;16)(p13;q22): results from CALGB 8461. J Clin Oncol. 2004;22(6):1087–94.
Döhner H, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–47.
Walker AR. How to approach shared decision making when determining consolidation, maintenance therapy, and transplantation in acute myeloid leukemia. Hematology Am Soc Hematol Educ Program. 2020;2020(1):51–6.
Zhang XH, et al. The consensus from The Chinese Society of Hematology on indications, conditioning regimens and donor selection for allogeneic hematopoietic stem cell transplantation: 2021 update. J Hematol Oncol. 2021;14(1):145.
Bhansali RS, Pratz KW, Lai C. Recent advances in targeted therapies in acute myeloid leukemia. J Hematol Oncol. 2023;16(1):29.
Halpern AB, et al. Phase 1/2 study of sorafenib added to cladribine, high-dose cytarabine, G-CSF, and mitoxantrone in untreated AML. Blood Adv. 2023;7(17):4950–61.
Acknowledgements
We thank all the patients for their participation and the researchers for their hard work.
Funding
This work was supported by grants from the Distinguished Taishan Scholars in Climbing Plan (tspd20210321), the Distinguished Taishan Scholars Plan (tstp20230653) and the National Natural Science Foundation of China (82070160, 82370165, 82270174, 82100176).
Author information
Authors and Affiliations
Contributions
MJ, JY and CJ conceived and designed the study. YH and WL analyzed the data and wrote the paper. HJ and JW collected data. WL, YZ, RJ, FL, SZ, CZ and SJ provided suggestions for paper writing. All authors reviewed and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This retrospective study involved all procedures in compliance with the requirements of the Ethics Committee.
Consent for publication
All authors of this article agree to publish in your journal.
Competing interests
The authors have no conflicts of interest related to this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hao, Y., Ji, M., Jin, S. et al. Medium-cumulative dose of cytarabine in consolidation therapy shows the greatest benefit in AML patients. Holist Integ Oncol 3, 22 (2024). https://doi.org/10.1007/s44178-024-00088-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44178-024-00088-7