Abstract
Background
Cytarabine based therapy has been the standard consolidation regimen for AML (acute myeloid leukemia) for decades. However, the optimal dose, regimen and schedule is not known. HIDAC (high dose cytarabine at 18 g/m2) has been the conventional standard, however, recent studies have shown that intermediate doses of cytarabine (IDAC) have equal efficacy and lesser toxicities.
Methods
We retrospectively analysed 75 AML patients who entered consolidation out of 167 patients who underwent induction therapy between 2014 and 2018. HIDAC (at 18 g/m2) was given to 39 patients and 36 patients received IDAC at 9 g/m2.
Results
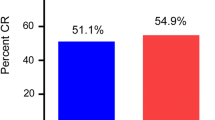
Median age was 28 years (range 2–60). Male: female ratio was 1.02. More courses were administered in out-patient setting in IDAC group 61% (n = 58/95 courses) than in HIDAC 29% (n = 29/101 courses); p < 0.001. Incidence of clinically documented infection (CDI) was higher in HIDAC group (23.7%) compared to IDAC (8.4%), p = 0.004, and diarrhea showed a higher trend in the HIDAC group (9.9% vs. 3.1%; p = 0.052). Other toxicities were similar in both groups. There were 4 consolidation deaths in HIDAC whereas 3 deaths in IDAC group (p = 0.775). Median OS was 19.7 vs. 16.2 months and 3 years OS was 49.1% vs. 34.7% in HIDAC and IDAC groups respectively (p = 0.570). Median LFS was 12.6 versus 11.8 months; 3 years LFS was 46.4% versus 31% in HIDAC and IDAC groups respectively (p = 0.278).
Conclusion
For AML consolidation IDAC had lesser toxicity when compared with HIDAC though comparable efficacy needs to be confirmed with longer follow up and with prospective studies.


Similar content being viewed by others
Data Availability
Will be made available on request.
Code Availability
Not applicable.
References
Schlenk RF (2014) Post-remission therapy for acute myeloid leukemia. Haematologica 99:1663–1670
Schaich M, Röllig C, Soucek S et al (2011) Cytarabine dose of 36 g/m2 compared with 12 g/m2 within first consolidation in acute myeloid leukemia: results of patients enrolled onto the prospective randomized AML96 study. J Clin Oncol Off J Am Soc Clin Oncol 29:2696–2702
Döhner H, Estey E, Grimwade D et al (2017) Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 129:424–447
Hengeveld M, Suciu S, Karrasch M et al (2012) Intensive consolidation therapy compared with standard consolidation and maintenance therapy for adults with acute myeloid leukaemia aged between 46 and 60 years: final results of the randomized phase III study (AML 8B) of the European Organization for Research and Treatment of Cancer (EORTC) and the Gruppo Italiano Malattie Ematologiche Maligne dell’Adulto (GIMEMA) Leukemia Cooperative Groups. Ann Hematol 91:825–835
Tallman MS, Gilliland DG, Rowe JM (2005) Drug therapy for acute myeloid leukemia. Blood 106:1154–1163
Milligan DW, Grimwade D, Cullis JO et al (2006) Guidelines on the management of acute myeloid leukaemia in adults. Br J Haematol 135:450–474
Keating S, Suciu S, de Witte T et al (1996) Prognostic factors of patients with acute myeloid leukemia (AML) allografted in first complete remission: an analysis of the EORTC-GIMEMA AML 8A trial. The European Organization for Research and Treatment of Cancer (EORTC) and the Gruppo Italiano Malattie Ematologiche Maligne dell’ Adulto (GIMEMA) Leukemia Cooperative Groups. Bone Marrow Transpl 17:993–1001
Mayer RJ, Davis RB, Schiffer CA et al (1994) Intensive postremission chemotherapy in adults with acute myeloid leukemia. Cancer and Leukemia Group B. N Engl J Med 331:896–903
Sperr WR, Piribauer M, Wimazal F et al (2004) A novel effective and safe consolidation for patients over 60 years with acute myeloid leukemia: intermediate dose cytarabine (2 × 1 g/m2 on days 1, 3, and 5). Clin Cancer Res 10:3965–3971
van Prooijen HC, Dekker AW, Punt K (1984) The use of intermediate dose cytosine arabinoside (ID Ara-C) in the treatment of acute non-lymphocytic leukaemia in relapse. Br J Haematol 57:291–299
Löwenberg B (2013) Sense and nonsense of high-dose cytarabine for acute myeloid leukemia. Blood 121:26–28
Fopp M, Fey MF, Bacchi M et al (1997) Post-remission therapy of adult acute myeloid leukaemia: one cycle of high-dose versus standard-dose cytarabine. Ann Oncol 8:251–257
Medeiros BC, Chan SM, Daver NG et al (2019) Optimizing survival outcomes with post-remission therapy in acute myeloid leukemia. Am J Hematol 94:803–811
Miyawaki S, Ohtake S, Fujisawa S et al (2011) A randomized comparison of 4 courses of standard-dose multiagent chemotherapy versus 3 courses of high-dose cytarabine alone in postremission therapy for acute myeloid leukemia in adults: the JALSG AML201 Study. Blood 117:2366–2372
Jaramillo S, Benner A, Krauter J et al (2017) Condensed versus standard schedule of high-dose cytarabine consolidation therapy with pegfilgrastim growth factor support in acute myeloid leukemia. Blood Cancer J 7:e564
Philip C, George B, Ganapule A et al (2015) Acute myeloid leukaemia: challenges and real world data from India. Br J Haematol 170:110–117
Chauhan PS, Ihsan R, Singh LC et al (2013) Mutation of NPM1 and FLT3 genes in acute myeloid leukemia and their association with clinical and immunophenotypic features. Dis Markers 35:581–588
Bahl A, Sharma A, Raina V et al (2015) Long-term outcomes for patients with acute myeloid leukemia: a single-center experience from AIIMS, India. Asia Pac J Clin Oncol 11:242–252
Cancer Statistics Review, 1975–2014—SEER Statistics. In: SEER. Accessed from: Leukemia, CSR 1975–2014 (cancer.gov)—SEER Statistics on 10th October 2019.
Craig BM, Rollison DE, List AF, Cogle CR (2012) Underreporting of myeloid malignancies by United States Cancer Registries. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 21:474–481
Juliusson G, Lazarevic V, Hörstedt A-S et al (2012) Acute myeloid leukemia in the real world: why population-based registries are needed. Blood 119:3890–3899
Estey EH (2018) Acute myeloid leukemia: 2019 update on risk-stratification and management. Am J Hematol 93(10):1267–1291
Thomas X, Elhamri M, Raffoux E et al (2011) Comparison of high-dose cytarabine and timed-sequential chemotherapy as consolidation for younger adults with AML in first remission: the ALFA-9802 study. Blood 118:1754–1762
Fukushima T, Urasaki Y, Yamaguchi M et al (2012) A randomized comparison of modified intermediate-dose Ara-C versus high-dose Ara-C in post-remission therapy for acute myeloid leukemia. Anticancer Res 32:643–647
Burnett AK, Russell NH, Hills RK et al (2013) Optimization of chemotherapy for younger patients with acute myeloid leukemia: results of the medical research council AML15 trial. J Clin Oncol Off J Am Soc Clin Oncol 31:3360–3368
Kim DS, Kang K-W, Lee SR et al (2015) Comparison of consolidation strategies in acute myeloid leukemia: high-dose cytarabine alone versus intermediate-dose cytarabine combined with anthracyclines. Ann Hematol 94:1485–1492
Abdul Halim NA, Wong GC, Aloysius HY et al (2016) High dose cytarabine is superior to intermediate dose cytarabine as post-remission therapy for younger patients with favorable risk acute myeloid leukemia. Blood 128:4032–4032
Kolla B, Halim NAA, Sachs Z et al (2019) High risk of relapse with intermediate dose cytarabine for consolidation in young favorable risk AML patients following induction with 7+3. Blood 134:3432–3432
Wei H, Wang Y, Gale RP et al (2020) Randomized trial of intermediate-dose cytarabine in induction and consolidation therapy in adults with acute myeloid leukemia. Clin Cancer Res 26:3154–3161
Wu D, Duan C, Chen L, Chen S (2017) Efficacy and safety of different doses of cytarabine in consolidation therapy for adult acute myeloid leukemia patients: a network meta-analysis. Sci Rep 7:1–10
Kalaiyarasi JP, Ganesan P, Kannan K et al (2019) Outcomes of intensive treatment of adult acute myeloid leukemia patients: a retrospective study from a single centre. Indian J Hematol Blood Transfus 35:248–254
Magina KN, Pregartner G, Zebisch A et al (2017) Cytarabine dose in the consolidation treatment of AML: a systematic review and meta-analysis. Blood 130:946–948
Acknowledgements
Authors thank all the staffs of department of Medical Oncology for completion of this work. We acknowledge the contribution of JIPMER (Jawaharlal Institute of Postgraduate Medical Education and Research) for providing logistic support for this work.
Funding
No specific funding was involved in conduct of this retrospective study.
Author information
Authors and Affiliations
Contributions
Study conceptualization & methodology: SK, HS, DR, BD. Data collection and analysis: DR, HS, SK, AC, AB. Manuscript writing: DR, HS, AB, SK, PG. Review and editing: SK, HS, AC, BD, PG. Final approval of manuscript: by all authors.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Ethics Approval
Institute Ethics approval was taken before commencement of the study.
Consent to Participate
Waiver of consent was granted for the retrospective data and analysis.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ravikumar, D., Saju, H., Choudary, A. et al. Outcomes of HIDAC 18 g Versus IDAC 9 g in Consolidation Therapy of Acute Myeloid Leukemia: A Retrospective Study. Indian J Hematol Blood Transfus 38, 31–41 (2022). https://doi.org/10.1007/s12288-021-01430-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12288-021-01430-z