Abstract
Lung cancer is the most prevalent cancer in the world, and the main treatment for advanced non-small cell lung cancer is immunotherapy combined with chemotherapy. In recent years, bTMB has received increasing attention as an emerging metric for monitoring the efficacy of tumour immunotherapy in terms of its operability, accessibility and real-time nature. We envisaged whether immunotherapy alone could be used to reduce the side effects of chemotherapy in patients with high bTMB lung cancer. We thus did a meta-analysis in order to show that immunotherapy alone is feasible in patients with high bTMB NSCLC.
Methods This study aims to compare the efficacy of PD- 1/PD-L1 inhibitors (namely, atezolizumab, pembrolizumab, nivolumab, or tislelizumab) versus chemotherapy in NSCLC patients. The search for relevant studies was conducted in three major databases (i.e., PubMed, Embase, and Medline) up until January 2023. Specifically, we identified studies that reported risk ratios (HRs) for reporting progression-free survival (PFS) or overall survival (OS), or objective remission rates (ORs) for immunotherapy alone versus chemotherapy in high bTMB and low bTMB patient groups. Given that NSCLC represents the predominant type of lung cancer, we exclusively focused on this subtype. Our analysis encompassed a meta-analysis of the identified literature, incorporating heterogeneity analysis and sensitivity analysis. The quality of the evidence is evaluated using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to ascertain the reliability and robustness of the findings.
Result-We conducted a meta-analysis of seven randomised controlled trials including 4,755 patients with advanced non-small cell lung cancer (NSCLC) evaluated the efficacy of PD- 1 or PD-L1 monotherapy compared to chemotherapy alone. All patients were randomized to receive either PD- 1/PD-L1 treatment alone or chemotherapy alone as a control. In the high bTMB patient group, PD- 1/PD-L1 monotherapy resulted in significant improvements in overall survival (HR = 0.55, 95% CI 0.49–0.61, p = 0.77) and progression-free survival (HR = 0.74, 95% CI 0.68–0.81, p = 0.78) compared to chemotherapy alone. Conversely, in the low bTMB patient group, PD- 1 monotherapy or PD-L1 monotherapy failed to demonstrate significant improvements in overall survival (HR = 0.82, 95% CI 0.73–0.92, p = 0. 13) and progression-free survival (HR = 1.22, 95% CI 1.22- 1.45, p = 0.003) in advanced NSCLC.
Conclusion Our analysis suggests that monotherapy with immunotherapy is a feasible option for patients with advanced NSCLC and high bTMB. However, the results have to be construed with caution because of the small sample size and the potential bias in the studies included. Therefore, further research with larger sample sizes and rigorous study designs is necessary to confirm the observed benefits of immunotherapy in this patient population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Lung cancer represents a major public health concern worldwide, with the highest incidence of all cancers [1]. In China, it has the highest incidence and mortality rates among all malignancies, with NSCLC accounting for about 85% of cases. While surgical resection is the primary treatment for resectable NSCLC, only a minority of patients (20%-25%) are eligible for curative surgery. Even after undergoing surgery, long-term survival rates are suboptimal, with 5-year survival rates ranging from 36% to 60%. Chemotherapy remains the cornerstone of treatment for both advanced primary and metastatic lung cancer, although it has some limitations and adverse effects that hinder patient compliance, such as lack of tumor specificity and development of drug resistance. In the most recent guidelines, chemotherapy in combination with immunotherapy is recommended as a first-line treatment for advanced NSCLC [2], offering a promising therapeutic approach to improve patient outcomes. Over the past decade, the use of immune checkpoint inhibitors (ICIs) in the treatment of NSCLC has changed the treatment outlook for this stubborn disease [3]. In advanced NSCLC, an increasing number of clinical studies have shown that the use of ICIs can lead to major breakthroughs in PFS and OS [4,5,6]. As a result, the US Food and Drug Administration has been quick to include ICIs in the first-line treatment of advanced NSCLC [7]. PD- 1 immunotherapy, which involves the programmed death inhibitory factor 1 (PD- 1) and its ligand PD-L1, is currently a popular medical treatment. PD- 1 is a programmed death molecule that binds to receptors on immune cells, specifically T cells, and PD-L1, the ligand, is present on tumour cells. When the two combine, the T cell's ability to kill tumour cells is diminished. Blocking antibodies to PD- 1 or PD-L1 have been made and they have since been approved for the treatment of a number of advanced cancers, including NSCLC, making them successful ICIs. In comparison to chemotherapy and radiotherapy, immunotherapy has shown significant improvements in durable remission rates in monotherapy and combination therapy for patients who have advanced NSCLC, extending long-term survival while also limiting adverse effects. An emerging biomarker to monitor its impact is the tumour mutation burden (TMB), which is defined as the quantity of mutations present in a tumour. It may promote the creation of new antigens and further increase immunogenicity. Recently, studies have demonstrated that TMB which measured by whole exome sequencing (WES) or next-generation sequencing (NGS) of the cancer genome (CGP), can be a effective candidate biomarker for clinical outcomes in melanoma, lung cancer, and uroepithelial carcinomas when using immune checkpoint blockage (ICBs) [8]. TMB is a measure of the amount of tumor mutation, and a higher TMB is associated with increased neoantigen formation, improved recognition by T-cells, and better clinical outcomes with immune checkpoint inhibitors (ICIs). Despite TMB being the most commonly used biomarker for ICIs, it has several limitations, including the need for biopsy, which is not always feasible in approximately 30% of NSCLC patients. Consequently, non-invasive detection of TMB based on circulating tumor DNA (ctDNA) in blood has emerged as an alternative method for monitoring prognosis. With the advent of liquid biopsy and the next-generation sequencing (NGS) technologies, the first EGFR mutation-based liquid biopsy products were approved by the FDA in 2016 [9]. Recently, a study by scientists from UC Davis, Foundation Medicine, and other institutions validated the value of blood tumor mutational burden (bTMB) for OS and PFS in NSCLC patients treated with immunotherapy. While chemotherapy-immunotherapy combinations are not effective for all solid tumors, studies involving PD-L1 positive patients have demonstrated that atezolizumab and cemiplimab, immunotherapy agents, are more effective than chemotherapy. In addition, in a randomized study involving partially advanced unresectable stage NSCLC patients, durvalumab showed a noticeable improvement in median PFS compared to placebo, without progression following standard concurrent platinum cytotoxic and radiotherapy treatment [10, 11]. The notion of utilizing mono-immunotherapy for select patients with NSCLC has emerged as a potential strategy. PD-L1 expression as the most commonly used biomarker for immunotherapy. cps scoring is a type of scoring for tumor immunity. However, its sensitivity and specificity may vary for different cancers [12]. Considering the prognostic significance of bTMB in immunotherapy, we were prompted to investigate the feasibility of implementing immunotherapy alone as an alternative treatment option for patients with elevated bTMB [13]. The objective was to mitigate the adverse effects of chemotherapy, alleviate cancer-related conditions and improve patients' quality of life, and attain favorable therapeutic outcomes. To this end, we conducted a meta-analysis.
2 Conclusion
The feasibility of utilizing immunotherapy alone for treating advanced NSCLC patients with high bTMB has been demonstrated. However, the reliability of the results is subject to various biases and limitations inherent in the small sample size utilized.
2.1 Search strategy
The included literature was independently searched by two investigators for databases such as PubMed, Embase and Medline. From inception to January 2023 RCTs. The major search terms were as follows: (lung cancer [MeSH Major Topic]) AND (blood tumor mutation burden)
2.2 Study selection
The randomized controlled trials (RCTs) included in this study satisfied the following criteria: (1) patients with advanced NSCLC who provided a bTMB cut-off value, which divided them into a high bTMB group and a low bTMB group, and supplied sample sizes along with calculated hazard ratios (HRs) and 95% confidence intervals (CIs); (2) all patients were received with immunotherapy, such as PD1, PD-L1 inhibitors, and ctla inhibitors; (3) the control group was treated with chemotherapy and standard care; and (4) bTMB testing was conducted on all patients before treatment. Literature reviews, letters, trial designs, editorials, conference abstracts and unrelated clinical trials were exempted. Trials that lacked bTMB cut-off values or did not use chemotherapy as a control, as well as studies that reported efficacy analyses only in subgroups of patients with high or low bTMB, were excluded. Next-generation sequencing (NGS) genomic assays were utilized for bTMB testing in all literature. The direct groupings of high and low bTMB used in this study were based on the included literature, which employed varying optimal cut-off values for bTMB. As there is no standardised criteria for grouping high bTMB versus low bTMB, the groupings regarding high and low bTMB in this study were directly adopted from the original groupings in the included literature, and data from the included literature were used. It is because the values for high and low expression of bTMB are not uniformly specified, and in the literature we included, such as Yu-tong Chen and Gandara and Yiting Dong [14,15,16] who performed similar analyses of two well-known clinical trials (POPLAR and OAK), although they studied from the same trials, they included different numbers of NSCLC patients with different groupings and cut-off values for high and low bTMB, and their findings are still important for our mete-analysis.
2.3 Data extraction
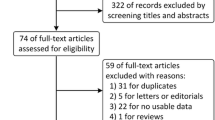
The included studies were analyzed for relevant information. The extracted data included the name of the author, year of publication of the literature, type of tumour studied, type of study and method, type of blood biomarker, method of biomarker detection, cut-off point for blood biomarkers, type of immune checkpoint inhibitor (ICI) used, type of outcome, and results (HRs and 95% CI). After conducting the database search, 40 articles were retrieved. Using the exclusion criteria specified, seven articles that were not relevant to the study were excluded. Specifically, one duplicate article, five reviews, four articles not related to lung cancer, 13 articles without a bTMB cut-off, two articles with missing experimental results, and two articles that did not compare immunotherapy alone with chemotherapy alone were removed. As a result, our meta-analysis included seven articles (Figure 1).
Two independent investigators extracted the following information from each trial: year of trial publication, first author of each trial, trial design method, numbers of patients with high and low bTMB in the trial and control groups, treatment regimens in both groups, treatment routes, bTMB cut-off values, and outcomes for patients in the high bTMB and low bTMB groups using immune agents compared with chemotherapy, including 95% CI, PFS, and OS. Any discrepancies were resolved through consensus among the investigators.
2.4 Quality assessment
We used Cochrane's own risk of bias tool to assess the quality of the included RCTs. The tool assesses the risk of bias for each RCT based on six criteria, namely random sequence generation(selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other biases. Each criterion was evaluated and categorized as low risk, high risk, or unclear risk. The results of the quality assessment are depicted in Figure 2. Two investigators independently performed data extraction and quality assessment, and any discrepancies were resolved through discussion between all investigators.
2.5 Statistical analysis
The study evaluated the differences curative effect between PD- 1/PD-L1 inhibitors alone and chemotherapy (CT) for high bt and low bTMB groups using hazard ratios (HR) for OS and PFS. To assess heterogeneity between studies, a q-test was performed, and the I2 and Q-value statistics were used, with considerable heterogeneity defined as I2 > 50% and/or p < 0. 1. Conversely, non-heterogeneity was defined as I2 < 50% and/or p > 0. 1. The overall effect was deemed to be statistically significant at p < 0.05. A series of sensitivity analyses were performed on subgroups with high heterogeneity to confirm the robustness of the results. Asymmetric funnel plots were used to remove studies with heterogeneity, and Review Manager 5.3 was used to conduct all analyses.
A total of 4755 patients with NSCLC were included from 7 trials (13 cohorts) [14,15,16,17,18,19,20] to examine the association between OS and PFS in patients with high bTMB receiving immunotherapy versus chemotherapy. In comparison to chemotherapy, PD- 1/PD-L1 inhibitors alone significantly extended OS (HR=0.55, 95% CI 0.49-0.61, p=0.77) and PFS (HR=0.74, 95% CI 0.68-0.81, p=0.78) in patients with high bTMB. Conversely, in patients with low bTMB, immunotherapy alone did not demonstrate any significant improvement in the risk of death in advanced NSCLC (OS: HR=0.82, 95% CI 0.73-0.92, p=0. 13 and PFS: HR= 1.22, 95% CI 1.02- 1.45, p=0.003). In patients with high bTMB, the effect of PD- 1/PD-L1 inhibitors alone on OS in NSCLC (I2 = 64%, P=0.0009) was more heterogeneous (Figure 3), but after we removed the two studies from Peters. S, we found (I2= 0%, P=0.77) that the heterogeneity became less, and from the original article we found that this cohort was an intention-to-treat population, which may have late cancer stage and drug treatment is no longer effective, among other reasons, so we removed this cohort from our analysis for Peters. S. We will also use a funnel diagram below to explain the rationale for removing the study. In patients with high bTMB, the effect of PD- 1/PD-L1 inhibitors alone on PFS in NSCLC (I2= 0%, P=0.78) was less heterogeneous in this group and the results were more reliable (Figure 4). However, there was a moderate degree of heterogeneity in the outcome of PFS in NSCLC patients with low bTMB using PD- 1/PD-L1 inhibitors alone (I2=67%, P=0.003). There was a mild degree of heterogeneity in OS outcomes in NSCLC patients with low bTMB using PD- 1/PD-L1 inhibitors alone (I2=36%, P=0.13) (Figure 5).
2.6 Risk of bias and sensitivity analysis
All trials were randomised and controlled, so the risk of selective bias in Random sequence generation (selection bias) was low. The lack of blinding was a major issue affecting quality. This was because most of the experiments are open-label.
2.7 Relationship between high bTMB level and overall survival and progression-free survival
In advanced NSCLC, a growing number of clinical studies have shown that the use of ICIs can lead to significant breakthroughs in PFS and OS, and in this way we focus on the OS and PFS of immunotherapy alone compared to chemotherapy [4, 5, 13]. Six trials (13 cohorts) assessed the relationship between OS with PD- 1/PD-L1 inhibitors alone and PFS with PD- 1/PD-L1 inhibitors in patients with high bTMB. Six trials (12 cohorts) assessed the relationship between PFS with PD- 1/PD-L1 inhibitors alone and PFS with PD- 1/PD-L1 inhibitors in patients with high bTMB. We graded quality using GRADE software and all results were of high quality, for the results showed that in patients with high bTMB, the use of immunotherapy alone significantly reduced the risk of death in patients with NSCLC (OS: HR=0.61 95% CI 0.57-0.69 I2=64%, P=0.0009, PFS: HR=0.74 95% CI 0.68 -0.81 I2 =0%, P=0.78). However, in patients with high bTMB, the I2=64% effect of immune agents alone on OS was moderately heterogeneous, and the effect of immune agents alone on PFS was minimally heterogeneous. We therefore performed a sensitivity analysis of the effect of immune agents alone on OS in patients with high bTMB. In the funnel plot, the two studies of Peters, S [18] were on one side of the axis of symmetry and one study was even on the outside side of the funnel plot, suggesting that there may be a large error in these two studies (Figure 6), so we removed these two studies. After removing this study, we obtained OS for immunotherapy alone versus chemotherapy alone in patients with high bTMB (HR=0.55, 95% CI 0.49-0.61, p=0.77, I2=0%), demonstrating the error of Peters,S on the effect of immunization alone on OS in patients with high bTMB, and therefore our study removed Peters,S on the effect of immunization alone on OS in patients with high bTMB. The results are reliable because I2=0%, p=0.77 (>0. 1) and p<0.00001 (p<0.05 is statistically significant) in the test for overall effect. In conclusion, OS and PFS were better in patients with high bTMB treated with anti-PD- 1 or PD-L1 alone than in patients treated with CT alone.
2.8 Relationship between low bTMB level and overall survival and progression-free survival
In advanced NSCLC, we examined the impact of immune agents alone on OS and PFS in patients with low bTMB. Our analysis included six trials (9 cohorts) that investigated the relationship between OS in patients with low bTMB treated with immune agents alone, and six trials (8 cohorts) that assessed the relationship between PFS in patients with low bTMB treated with immune agents alone. Quality grading, performed using GRADE software, indicated high quality for all results. However, our findings revealed that the use of immune agents alone did not result in reduced risk of death in patients with low bTMB NSCLC (OS: HR=0.82, 95% CI 0.73-0.92, I2=36%, p=0. 13 and PFS: HR= 1.22, 95%CI 1.02- 1.45, I2=67%, p=0.003). The mild heterogeneity of I2=36% and p=0. 13 in OS was acceptable, as long as p is greater than 0. 1 and I2<50%. However, for PFS in patients with NSCLC with low bTMB, the effect of immunotherapy alone compared to chemotherapy alone was I2=67%, and p<0. 1, indicating large heterogeneity in this outcome. Additional studies are needed to elucidate the impact of immunotherapy in comparison to chemotherapy on PFS in patients with low bTMB.
2.9 Principal findings
The present meta-analysis demonstrates that immunotherapy alone significantly reduces the risk of death in NSCLC patients with high bTMB when compared to chemotherapy, with a 45% reduction in risk for OS and 26% reduction in risk for PFS. In contrast, for patients with low bTMB, the use of immune agents alone did not significantly decrease the risk of death, and significant heterogeneity was observed, likely because of the limited sample size. However, the current study may be limited by the small number of trials included, and divergent findings have been also reported in other researchs. For instance, Jun Lu reported that atezolizumab alone produced greater benefits in both PFS and OS in patients with low and high bTMB (≤7 or >20) in the OAK cohort, as opposed to POPLAR [21]. Further prospective trials are warranted to validate the effectiveness of immunotherapy alone in patients with high bTMB.
2.10 Advantages of bTMB and novel detection methods
Recent research has indicated that blood-based tumour mutation burden (bTMB) presents an appealing alternative to tissue-based TMB due to its numerous advantages. Firstly, bTMB testing is non-invasive, eliminating the need for an intrusive biopsy. Secondly, the mutation coverage provided by cfDNA analysis is more comprehensive than that obtained from a single-site tumour biopsy. Thirdly, bTMB testing has the potential to detect disease recurrence earlier than conventional diagnostic imaging. The principal disadvantage of TMB is that it necessitates a biopsy, which can lead to many patients with advanced cancer being unable to undergo the procedure. Furthermore, a single biopsy may not offer a complete mutational profile of the tumour, particularly in the presence of metastases. Certain metastases may not be visible on radiological scans, rendering them unassessable. However, bTMB overcomes this limitation. Analyzing tumour genomes by a simple blood sample offers clear advantages over collecting tissue biopsies. Blood serves as a readily accessible and concurrent source of diagnostic material, and bTMB testing is likely to be less susceptible to the underlying sampling bias which associated with one-site tissue biopsies. The numerous advantages of bTMB have sparked a surge of interest in the area of blood-based DNA testing, with several reliable techniques for detecting cell-free DNA (cfDNA) mutations now available. These techniques include digital droplet PCR, allele-specific PCR and panel-based NGS2. In addition, more recently been developed methods such as digital PCR (dPCR), droplet digital PCR (ddPCR), and magnetic bead emulsion amplification (beam) are utilized to analyze ctDNA. Sequencing methods include tagged AMplicon deep sequencing (TAM-Seq), Duplex sequencing , safe-sequencing (Safe-Seq), cancer personalized analysis deep sequencing (CAPP-Seq) and safe-sequencing (Safe-Seq) [22]. Cell-free DNA (cfDNA) plays a pivotal role in detecting blood tumour mutation burden (bTMB) in NSCLC by quantifying the number of somatic mutations present in the genomic coding region. Advances in technology have made the prognostication of bTMB for immunotherapy. A study conducted by Zhijie Wang, MD demonstrated that the NCC-GP150 [23], a bTMB estimation algorithm optimized for genome size, was feasible [24]. Additionally, the GuardantOMNI ctDNA platform was found to be feasible, accurate, and reproducible for quantifying bTMB in plasma samples, as demonstrated by the study conducted by Han Si [23]. Furthermore, Xi Chen's research indicated that the OncOScreen panel can be used to be employed for assessing both tissue-based TMB and bTMB. Our own research has confirmed the utility of bTMB as a prospective biomarker for identifying patients with advanced NSCLC who are more likely to derive benefit from ICIs. The field of bTMB measurement is expected to expand with the advent of new methods and platforms. The numerous advantages of bTMB have motivated our research direction. However, bTMB also has its limitations. For instance, bTMB exhibits high specificity but low sensitivity, likely owing to the proportionality between the amount of circulating tumour DNA and tumour volume. Furthermore, although bTMB assessment can be performed simultaneously with somatic mutation testing for cfDNA in patients with stage IV NSCLC, using only one NGS test, reflex to tissue-based TMB assessment may be necessary in cases of low bTMB values, which may preclude proper assessment of the immunotherapeutic effect. Additional limitations include low yield, a lack of standardized techniques, and insufficient validation data [25].
2.11 Limitations and future study directions
Our investigation demonstrated that immune agents alone improved OS and PFS compared to ct in patients with high bTMB, suggesting that immunotherapy alone can be a feasible option for patients who have advanced lung cancer. However, the optimal cut-off values for bTMB included in the study exhibited variability, and the results were controversial as to the precise bTMB cut-off value that yielded greater effectiveness of immunotherapy alone. In the Gandara study, the assay performed optimally at three cut-off values of bTMB, specifically bTMB≥ 10, ≥ 16, and ≥20 [15]. Han Si's study showed that high bTMB predicted the clinical benefit of dulvalizumab plus trametumab over chemotherapy using a new bTMB algorithm and an optimal bTMB cut-off of ≥20 mut/Mb [8]. A POPLAR and OAK study determined that the optimal bTMB cut-offpoint was 13. In Zhijie Wang's study, an HR of 0.39 (0. 18-0.84) was found for self bTMB greater than 6 compared to less than 6 [26]. A newly published prospective study (B-F1RST) evaluating the role of bTMB in predicting the efficacy of Atezolizumab in the first-line treatment of advanced NSCLC showed a significant absolute prolongation of PFS and OS in patients in the bTMB ≥ 16 mutations/Mb group compared to the bTMB < 16 mutations/Mb group (median PFS: 5.0 months vs Edward S. Kim and his colleagues came to the same conclusion [27], with patients with bTMB ≥ 16 having longer OS at long-term follow-up. In Xi Chen's study [24], HR=0.39 (0. 13- 1. 17) for bTMB ≥ 11 versus < 11. These suggest that in patients with NSCLC, those with high bTMB appear to have a higher survival rate and are much more likely to obtain a benefit from immunotherapy. Interestingly, in a study by Wei Nie [28], patients were divided into three groups of high bTMB (≥14 mutations/Mb), medium (8- 13 mutations/Mb), and low (≤7 mutations/Mb), resulting in OS In Wei Nie's study, the groups with high and low bTMB had prolonged OS compared to medium, which is different from our findings. However, this may provide a new direction for future studies as to whether bTMB is better than or as effective as chemotherapy alone in patients with advanced NSCLC between certain areas. Future studies could divide the bTMB into groups to get a more precise range of bTMB.
There are some limitations to our statistics, firstly we only included 7 trials with 13 cohorts. They were mainly from the Pumbed, Embase, and Medline databases; literature from other databases was not included at this time. Second, as each experiment measured different cut-off values of bTMB and different scholars selected different sample sizes for the same experimental data, new classifications of cut-off values of bTMB were also made, which may lead to greater heterogeneity and affect the reliability of this study. Thirdly, although new techniques for measuring bTMB continue to beinnovated, bTMB measurement techniques are still in their early stages and some errors are bound to exist between measured and specific values. For instance, significant factors affecting ctDNA shedding, such as EGFR or TP53 mutations or amplification tumour burden and visceral metastases, are likely to be unbalanced in clinically validated cohorts [29, 30]. Fourth: bTMB is subject to interference by a number of factors. A study by Zhijie Wang, MD found that the maximum somatic allele frequency (MSAF), i.e. frequency (MSAF), the MSAF of all somatic mutations in each sample detected through next-generation sequencing, in order to reflect the quantity of ctDNA in the blood. Due to the interference of MSAF, bTMB is unable to predict the benefit of immunotherapy on OS. Secondly, some tumour DNA does not flow into the blood sufficiently; samples with inadequate tumour shedding cannot be reliably used to measure bTMB, resulting in bTMB not being measured. Finally, A retrospective analysis of the POPLAR and OAK studies found for the first time a concordance between bTMB and tissue TMB (tTMB), with patients with bTMB ≥ 16 mut/Mb having longer PFS in more than 1,000 patients treated with atalizumab, and bTMB correlating with tTMB [15]. bTMB values may correlate with TMB, although previous studies have found a close connection between bTMB and TMB in cancer patients [18]. However, these studies failed to assess the overall concordance [31], and in addition the relationship between bTMB and TMB is currently unclear and controversial due to the many heterogeneities in tumours. Whereas TMB is associated with the effects of receiving PD- 1 or PD-L1, and few of the literature we included linked bTMB to TMB in trials, future trials may need to examine bTMB and TMB together. Although immune agents alone had longer OS and PFS values in our analysis in patients with NSCLC with high bTMB, their effectiveness needs to be identified in more trials. A large number of previous studies have shown and the latest guidelines remain that chemotherapy plus immunotherapy is the first line of treatment for patients with lung cancer.
For future development: currently some scholars have tried to derive other annotators to predict the prognosis of NSCLC immunotherapy based on bTMB, such as af-bTMB, and there are also many studies investigating its efficacy. Zhijie Wang, MD created and validated a new-type LAF-bTMB algorithm, which can be used as a viable predictor of OS, PFS and ORR in NSCLC patients following immunotherapy [32]. This provides a new direction for the efficacy of immunotherapy on NSCLC in the future. Thanks to technological updates we can also dynamically detect bTMB, and Tao Jiang investigated the relationship between bTMB dynamics (∆bTMB) and survival benefit [20] : for NSCLC patients, PFS was significantly shorter in patients with elevated or unchanged bTMB levels after pretreatment (∆bTMB ≥ 0). Optimised bTMB obtained by excluding high frequency mutations (AF>5%) or ultra-low frequency mutations [AF/MSAF < 10%] had a higher predictive effect than conventional bTMB. We have demonstrated in this study the effect of immunotherapy alone in patients with high bTMB. In addition, when a patient has a low bTMB value, it may also have some other suggestive effects. For example, a low bTMB result may also be a result of low tumour load early in the disease as well as poor tumour shedding [33]. Future studies need to perform more subgroup analysison the basis of NSCLC, e.g. NSCLC is classified as squamous carcinoma , adenocarcinoma, large cell lung cancer, etc. There are several groups of mutations common to non-small cells EGFR, ROS1, ALK, etc., for which subgroup analyses can be performed, as well as subgroup analyses of ethnicity, age, whether or not one smokes, etc., to draw more precise conclusions. In addition, btmb is also used as an indicator to monitor the effectiveness of its treatment in other cancers, such as liver cancer and colon cancer [34, 35]. In addition, some scholars have investigated the relationship between chemotherapy combined with immunotherapy and immunization alone in patients with high Btmb NSCLC, e.g., the study by Leighl, N. B demonstrated that chemotherapy combined with immunotherapy resulted in longer OS and PFS compared to immunotherapy alone in patients with high bTMB NSCLC [36], but due to reasons such as a relatively small sample size, this requires future further studies. In addition, for some NSCLC patients with low bTMB, the most suitable treatment method also needs further research.
3 Discussion
Multiple studies have evidenced that the use of immune combination chemotherapy confers a substantial extension of OS and PFS in patients with advanced NSCLC when compared to chemotherapy alone [36, 37]. Our study has similarly demonstrated a noteworthy enhancement of OS and PFS in patients possessing high bTMB who were treated with PD- 1/PD-L1 inhibitors as opposed to chemotherapy. This treatment modality represents a novel avenue of treating NSCLC in the future, eschewing the use of chemotherapy in favor of immunotherapy. By avoiding chemotherapy, patients can avoid the painful and undesirable side effects associated with it, thereby reducing the likelihood of treatment abandonment due to intolerance. Our findings suggest that a chemotherapy-free treatment paradigm may be a viable option for NSCLC patients who are unable to tolerate chemotherapy but exhibit high bTMB. Nevertheless, given the limited size of our study cohort, further experimental validation is necessary to corroborate these findings. In particular, the optimal bTMB cutoff value warrants examination by future investigators. However, there are limitations to this paper, such as the insufficient sample size included and the optimal bTMB cutoff value is not clearly defined, which may shed some light on future directions.
4 Conclusion
The results of this meta-analysis suggest that the efficacy of PD- 1/PD-L1 inhibitors in the treatment of NSCLC may be related to bTMB levels. Patients with high bTMB treated with PD- 1/PD-L1 inhibitors alone had significantly improved OS and PFS compared with chemotherapy, whereas patients with low bTMB treated with immunotherapy alone had no significant improvement in OS. Immunotherapy alone is feasible in some NSCLC with high bTMB that are intolerant to chemotherapy.
Availability of data and materials
Not applicable.
Abbreviations
- NSCLC:
-
Non-small cell lung cancer
- OS:
-
Overall survival
- PFS:
-
Progress free survival
- ICIs:
-
Immune checkpoint inhibitors
- BTMB:
-
Blood tumor mutation burden
- TMB:
-
Tumor mutation burden
- HR:
-
Risk ratio
- ctDNA:
-
Circulating tumor DNA
- cfDNA:
-
Cell-free DNA
References
Panunzio A, Sartori P. Lung cancer and radiological imaging [J]. Curr Radiopharm. 2020;13(3):238–42.
Liu L, Bai H, Wang C, et al. Efficacy and safety of first-line immunotherapy combinations for advanced NSCLC: a systematic review and network meta-analysis [J]. J Thorac Oncol. 2021;16(7):1099–117.
Sato Y. Clinical utility of liquid biopsy-based companion diagnostics in the non-small-cell lung cancer treatment [J]. Explor Target Antitumor Ther. 2022;3(5):630–42.
Antonia SJ, Villegas A, Daniel D, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC[J]. N Engl J Med. 2018;379(24):2342–50.
Edward B, Garon M. Five-year overall survival for patients with advanced non-small-cell lung cancer treated with Pembrolizumab: results from the phase I KEYNOTE-001 study [J]. J Clin Oncol. 2019;37:2518.
Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in advanced squamous-cell non-small-cell lung cancer [J]. N Engl J Med. 2015;373(2):123–35.
Zhai X, Zhang J, Tian Y, et al. The mechanism and risk factors for immune checkpoint inhibitor pneumonitis in non-small cell lung cancer patients [J]. Cancer Biol Med. 2020;17(3):599–611.
Si H, Kuziora M, Quinn KJ, et al. A blood-based assay for assessment of tumor mutational burden in first-line metastatic NSCLC treatment: results from the MYSTIC Study [J]. Clin Cancer Res. 2021;27(6):1631–40.
Fridland S, Choi J, Nam M, et al. Assessing tumor heterogeneity: integrating tissue and circulating tumor DNA (ctDNA) analysis in the era of immuno-oncology - blood TMB is not the same as tissue TMB[J]. J Immunother Cancer. 2021;9(8):e002551.
Herbst RS, Giaccone G, De Marinis F, et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC[J]. N Engl J Med. 2020;383(14):1328–39.
Sezer A, Kilickap S, Gumus M, et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial[J]. Lancet. 2021;397(10274):592–604.
De Marchi P, Leal LF, Duval Da Silva V, et al. PD-L1 expression by Tumor Proportion Score (TPS) and Combined Positive Score (CPS) are similar in non-small cell lung cancer (NSCLC) [J]. J Clin Pathol. 2021;74(11):735–40.
Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer [J]. N Engl J Med. 2017;377(20):1919–29.
Dong Y, Zhu Y, Zhuo M, et al. Maximum somatic allele frequency-adjusted blood-based tumor mutational burden predicts the efficacy of immune checkpoint inhibitors in advanced non-small cell lung cancer [J]. Cancers (Basel). 2022;14(22):5649.
Gandara DR, Paul SM, Kowanetz M, et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab[J]. Nat Med. 2018;24(9):1441–8.
Chen YT, Seeruttun SR, Wu XY, et al. Maximum somatic allele frequency in combination with blood-based tumor mutational burden to predict the efficacy of Atezolizumab in advanced non-small cell lung cancer: a pooled analysis of the randomized POPLAR and OAK studies [J]. Front Oncol. 2019;9:1432.
Nie W, Wang ZJ, Zhang K, et al. ctDNA-adjusted bTMB as a predictive biomarker for patients with NSCLC treated with PD-(L)1 inhibitors[J]. BMC Med. 2022;20(1):170.
Peters S, Dziadziuszko R, Morabito A, et al. Atezolizumab versus chemotherapy in advanced or metastatic NSCLC with high blood-based tumor mutational burden: primary analysis of BFAST cohort C randomized phase 3 trial [J]. Nat Med. 2022;28(9):1831–9.
Rizvi NA, Cho BC, Reinmuth N, et al. Durvalumab with or without Tremelimumab vs standard chemotherapy in first-line treatment of metastatic non-small cell lung cancer: the MYSTIC phase 3 randomized clinical trial [J]. JAMA Oncol. 2020;6(5):661–74.
Jiang T, Chen J, Xu X, et al. On-treatment blood TMB as predictors for camrelizumab plus chemotherapy in advanced lung squamous cell carcinoma: biomarker analysis of a phase III trial [J]. Mol Cancer. 2022;21(1):4.
Lu J, Wu J, Lou Y, et al. Blood-based tumour mutation index act as prognostic predictor for immunotherapy and chemotherapy in non-small cell lung cancer patients [J]. Biomark Res. 2022;10(1):55.
Nagasaka M, Uddin MH, Al-Hallak MN, et al. Liquid biopsy for therapy monitoring in early-stage non-small cell lung cancer [J]. Mol Cancer. 2021;20(1):82.
Herath S, Sadeghi Rad H, Radfar P, et al. The role of circulating biomarkers in lung cancer [J]. Front Oncol. 2021;11:801269.
Chen X, Fang L, Zhu Y, et al. Blood tumor mutation burden can predict the clinical response to immune checkpoint inhibitors in advanced non-small cell lung cancer patients [J]. Cancer Immunol Immunother. 2021;70(12):3513–24.
Mamdani H, Ahmed S, Armstrong S, et al. Blood-based tumor biomarkers in lung cancer for detection and treatment [J]. Transl Lung Cancer Res. 2017;6(6):648–60.
Wang Z, Duan J, Cai S, et al. Assessment of blood tumor mutational burden as a potential biomarker for immunotherapy in patients with non-small cell lung cancer with use of a next-generation sequencing cancer gene panel [J]. JAMA Oncol. 2019;5(5):696–702.
Kim ES, Velcheti V, Mekhail T, et al. Blood-based tumor mutational burden as a biomarker for atezolizumab in non-small cell lung cancer: the phase 2 B-F1RST trial [J]. Nat Med. 2022;28(5):939–45.
Nie W, Qian J, Xu MD, et al. A non-linear association between blood tumor mutation burden and prognosis in NSCLC patients receiving atezolizumab [J]. Oncoimmunology. 2020;9(1):1731072.
Lam VK, Zhang J. Blood-based tumor mutation burden: continued progress toward personalizing immunotherapy in non-small cell lung cancer [J]. J Thorac Dis. 2019;11(6):2208–11.
Abbosh C, Birkbak NJ, Swanton C. Early stage NSCLC - challenges to implementing ctDNA-based screening and MRD detection [J]. Nat Rev Clin Oncol. 2018;15(9):577–86.
Crisafulli G, Sartore-Bianchi A, Lazzari L, et al. Temozolomide treatment alters mismatch repair and boosts mutational burden in tumor and blood of colorectal cancer patients [J]. Cancer Discov. 2022;12(7):1656–75.
Wang Z, Duan J, Wang G, et al. Allele frequency-adjusted blood-based tumor mutational burden as a predictor of overall survival for patients with NSCLC treated with PD-(L)1 inhibitors[J]. J Thorac Oncol. 2020;15(4):556–67.
Schuurbiers M, Huang Z, Saelee S, et al. Biological and technical factors in the assessment of blood-based tumor mutational burden (bTMB) in patients with NSCLC [J]. J Immunother Cancer. 2022;10(2):e004064.
Grasselli J, Elez E, Caratu G, et al. Concordance of blood- and tumor-based detection of RAS mutations to guide anti-EGFR therapy in metastatic colorectal cancer [J]. Ann Oncol. 2017;28(6):1294–301.
Franses JW, Lim M, Burgoyne AM, et al. Profile and predictors of blood tumor mutational burden in advanced hepatocellular carcinoma [J]. Oncologist. 2022;27(11):e908–11.
Leighl NB, Laurie SA, Goss GD, et al. CCTG BR34: a randomized phase 2 trial of Durvalumab and Tremelimumab with or without platinum-based chemotherapy in patients with metastatic NSCLC [J]. J Thorac Oncol. 2022;17(3):434–45.
Liu SV, Reck M, Mansfield AS, et al. Updated Overall Survival and PD-L1 Subgroup Analysis of Patients With Extensive-Stage Small-Cell Lung Cancer Treated With Atezolizumab, Carboplatin, and Etoposide (IMpower133)[J]. J Clin Oncol. 2021;39(6):619–30.
Acknowledgements
Not applicable.
Funding
Funding for this work is supported by the Key Research and Development Project Institute of Hainan Province (ZDYF2023SHFZ117).
Author information
Authors and Affiliations
Contributions
Niansong Qian conceived the project, Qiu Xiaochen, Yang Qinna and Gao Shuyue collected the data, and Zhao Feiyu organized and wrote the data. Several authors agreed on the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Consent for publication bas been obtained from all authors of this manuscript.
Competing interests
This article has been handled by other editors and has undergone rigorous peer review, and the authors of this article declare that they have no known competing financial interests or personal relationships and have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, F., Qiu, X., Yang, Q. et al. Prospect of immunotherapy alone in patients with advanced NSCLC with high btmb: a review and a meta-analysis. Holist Integ Oncol 2, 44 (2023). https://doi.org/10.1007/s44178-023-00065-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44178-023-00065-6