Abstract
Background
Tissue tumor mutation burden (tTMB) assessed by whole-exome sequencing (WES), which has been regarded as the gold standard method of tTMB measurement, can predict the clinical benefits of immune checkpoint inhibitors (ICIs). Multiple studies have investigated the feasibility of utilizing large panels to evaluate TMB but have obtained conflicting results. Furthermore, whether blood TMB (bTMB) can also be a predictive biomarker in NSCLC has not been determined.
Methods
Fifty-six advanced NSCLC patients treated with ICIs were enrolled, including an exploratory cohort (n = 42) and a small independent validation cohort (n = 14). Next-generation sequencing was performed on tumor and plasma samples collected prior to ICI treatment using a panel consisting of 520 cancer-related genes (OncoScreen) to evaluate tTMB/bTMB. WES was also performed on tumor samples to serve as references.
Results
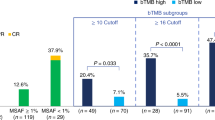
A positive correlation between tTMB derived from WES and OncoScreen was observed. OncoScreen-derived tTMB showed a positive correlation with OncoScreen-derived bTMB. Patients with OncoScreen-derived tTMB \(\ge\) 7 mutations/Mb (p = 0.003) or bTMB \(\ge\) 11 mutations/Mb (p = 0.0029) had superior progression-free survival (PFS). In the small validation cohort, patients with OncoScreen-derived bTMB \(\ge\) 11 mutations/Mb exhibited longer PFS (p = 0.192) with a nonsignificant difference. In all 42 patients who had available bTMB and PFS, patients with bTMB \(\ge\) 11 mutations/Mb had significantly longer PFS (p = 0.011) than those with bTMB \(<\) 11 mutations/Mb.
Conclusion
Our study confirmed the feasibility of using large panels to estimate TMB. We also demonstrated that bTMB can serve as a potential biomarker for predicting the efficacy of ICIs in NSCLC.





Similar content being viewed by others
Abbreviations
- TMB:
-
Tumor mutation burden
- WES:
-
Whole-exome sequencing
- ICI:
-
Immune checkpoint inhibitors
- NSCLC:
-
Non-small cell lung cancer
- PFS:
-
Progression-free survival
- DCB:
-
Durable clinical benefit
- PD-1:
-
Programmed cell death-1
- PD-L1:
-
Programmed cell death ligand-1
- MSI:
-
Microsatellite instability
- MMR:
-
DNA mismatch repair
- RECIST:
-
Response Evaluation Criteria in Solid Tumors
- PD:
-
Progressive disease
- ORR:
-
Overall response rate
- CR:
-
Complete response
- PR:
-
Partial response
- SD:
-
Stable disease
- NDB:
-
No durable benefit
- FFPE:
-
Formalin fixed paraffin embedded
- ctDNA:
-
Circulating tumor DNA
- AF:
-
Allele frequency
- SNP:
-
Single-nucleotide polymorphism
- SNV:
-
Single-nucleotide variant
- InDels:
-
Insertions and deletions
- ROC:
-
Receiver operating characteristic curves
- AUC:
-
Area under curve
- FDR:
-
False discovery rate
- AD:
-
Lung adenocarcinoma
- SCC:
-
Lung squamous cell carcinoma
- CNV:
-
Copy number variant
- ECOG PS:
-
Eastern Cooperative Oncology Group performance status
- CI:
-
Confidence interval
- HR:
-
Hazard ratio
- Mb:
-
Megabase
- OS:
-
Overall survival
References
Peters S, Gettinger S, Johnson ML, Janne PA, Garassino MC, Christoph D, Toh CK, Rizvi NA, Chaft JE, Carcereny Costa E, Patel JD, Chow LQM, Koczywas M, Ho C, Fruh M, van den Heuvel M, Rothenstein J, Reck M, Paz-Ares L, Shepherd FA, Kurata T, Li Z, Qiu J, Kowanetz M, Mocci S, Shankar G, Sandler A, Felip E (2017) Phase II Trial of atezolizumab as first-line or subsequent therapy for patients with programmed death-ligand 1-selected advanced non-small-cell lung cancer (BIRCH). J clin oncol : off J Am Soc Clin Oncol 35(24):2781–2789. https://doi.org/10.1200/jco.2016.71.9476
Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375(19):1823–1833. https://doi.org/10.1056/NEJMoa1606774
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhaufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crino L, Blumenschein GR Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373(17):1627–1639. https://doi.org/10.1056/NEJMoa1507643
Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Aren Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR (2015) Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373(2):123–135. https://doi.org/10.1056/NEJMoa1504627
Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, de Castro G, Jr., Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB, (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387(10027):1540–1550. https://doi.org/10.1016/S0140-6736(15)01281-7
Network NCC NCCN clinical practice guidelines in Oncology: Non-small cell lung cancer (Version 8.2020)
Vanderwalde A, Spetzler D, Xiao N, Gatalica Z, Marshall J (2018) Microsatellite instability status determined by next-generation sequencing and compared with PD-L1 and tumor mutational burden in 11,348 patients. Cancer Med 7(3):746–756. https://doi.org/10.1002/cam4.1372
Rizvi NA, Mazieres J, Planchard D, Stinchcombe TE, Dy GK, Antonia SJ, Horn L, Lena H, Minenza E, Mennecier B, Otterson GA, Campos LT, Gandara DR, Levy BP, Nair SG, Zalcman G, Wolf J, Souquet PJ, Baldini E, Cappuzzo F, Chouaid C, Dowlati A, Sanborn R, Lopez-Chavez A, Grohe C, Huber RM, Harbison CT, Baudelet C, Lestini BJ, Ramalingam SS (2015) Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 16(3):257–265. https://doi.org/10.1016/s1470-2045(15)70054-9
Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS (2014) Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515(7528):563–567. https://doi.org/10.1038/nature14011
Gettinger SN, Horn L, Gandhi L, Spigel DR, Antonia SJ, Rizvi NA, Powderly JD, Heist RS, Carvajal RD, Jackman DM, Sequist LV, Smith DC, Leming P, Carbone DP, Pinder-Schenck MC, Topalian SL, Hodi FS, Sosman JA, Sznol M, McDermott DF, Pardoll DM, Sankar V, Ahlers CM, Salvati M, Wigginton JM, Hellmann MD, Kollia GD, Gupta AK, Brahmer JR (2015) Overall survival and long-term safety of nivolumab (Anti-Programmed Death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J clin oncol : off j Am Soc Clin Oncol 33(18):2004–2012. https://doi.org/10.1200/jco.2014.58.3708
Mansfield AS, Murphy SJ, Peikert T, Yi ES, Vasmatzis G, Wigle DA, Aubry MC (2016) Heterogeneity of programmed cell death Ligand 1 expression in multifocal lung cancer. Clin cancer res : an off j Am Assoc Cancer Res 22(9):2177–2182. https://doi.org/10.1158/1078-0432.ccr-15-2246
Patel SP, Kurzrock R (2015) PD-L1 Expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther 14(4):847–856. https://doi.org/10.1158/1535-7163.mct-14-0983
Schumacher TN, Schreiber RD (2015) Neoantigens in cancer immunotherapy. Science (New York, NY) 348(6230):69–74. https://doi.org/10.1126/science.aaa4971
Riaz N, Havel JJ, Makarov V, Desrichard A, Urba WJ, Sims JS, Hodi FS, Martin-Algarra S, Mandal R, Sharfman WH, Bhatia S, Hwu WJ, Gajewski TF, Slingluff CL Jr, Chowell D, Kendall SM, Chang H, Shah R, Kuo F, Morris LGT, Sidhom JW, Schneck JP, Horak CE, Weinhold N, Chan TA (2017) Tumor and microenvironment evolution during immunotherapy with nivolumab. Cell 171(4):934–949. https://doi.org/10.1016/j.cell.2017.09.028
Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, Miller ML, Rekhtman N, Moreira AL, Ibrahim F, Bruggeman C, Gasmi B, Zappasodi R, Maeda Y, Sander C, Garon EB, Merghoub T, Wolchok JD, Schumacher TN, Chan TA (2015) Cancer immunology mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science (New York, NY) 348(6230):124–128. https://doi.org/10.1126/science.aaa1348
Yarchoan M, Hopkins A, Jaffee EM (2017) Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med 377(25):2500–2501. https://doi.org/10.1056/NEJMc1713444
Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, Chung HC, Kindler HL, Lopez-Martin JA, Miller WH Jr, Italiano A, Kao S, Piha-Paul SA, Delord JP, McWilliams RR, Fabrizio DA, Aurora-Garg D, Xu L, Jin F, Norwood K, Bang YJ (2020) Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol 21(10):1353–1365. https://doi.org/10.1016/s1470-2045(20)30445-9
Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, Schrock A, Campbell B, Shlien A, Chmielecki J, Huang F, He Y, Sun J, Tabori U, Kennedy M, Lieber DS, Roels S, White J, Otto GA, Ross JS, Garraway L, Miller VA, Stephens PJ, Frampton GM (2017) Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genom med 9(1):34. https://doi.org/10.1186/s13073-017-0424-2
Rizvi H, Sanchez-Vega F, La K, Chatila W, Jonsson P, Halpenny D, Plodkowski A, Long N, Sauter JL, Rekhtman N, Hollmann T, Schalper KA, Gainor JF, Shen R, Ni A, Arbour KC, Merghoub T, Wolchok J, Snyder A, Chaft JE, Kris MG, Rudin CM, Socci ND, Berger MF, Taylor BS, Zehir A, Solit DB, Arcila ME, Ladanyi M, Riely GJ, Schultz N, Hellmann MD (2018) Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J clin oncol : off j Am Soc Clin Oncol 36(7):633–641. https://doi.org/10.1200/JCO.2017.75.3384
Gandara DR, Paul SM, Kowanetz M, Schleifman E, Zou W, Li Y, Rittmeyer A, Fehrenbacher L, Otto G, Malboeuf C, Lieber DS, Lipson D, Silterra J, Amler L, Riehl T, Cummings CA, Hegde PS, Sandler A, Ballinger M, Fabrizio D, Mok T, Shames DS (2018) Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med 24(9):1441–1448. https://doi.org/10.1038/s41591-018-0134-3
Wang Z, Duan J, Cai S, Han M, Dong H, Zhao J, Zhu B, Wang S, Zhuo M, Sun J, Wang Q, Bai H, Han J, Tian Y, Lu J, Xu T, Zhao X, Wang G, Cao X, Li F, Wang D, Chen Y, Bai Y, Zhao J, Zhao Z, Zhang Y, Xiong L, He J, Gao S, Wang J (2019) Assessment of blood tumor mutational burden as a potential biomarker for immunotherapy in patients with non-small cell lung cancer with use of a next-generation sequencing cancer gene panel. JAMA Oncol 5(5):696–702. https://doi.org/10.1001/jamaoncol.2018.7098
Li L, Wang Y, Shi W, Zhu M, Liu Z, Luo N, Zeng Y, He Y (2019) Serial ultra-deep sequencing of circulating tumor DNA reveals the clonal evolution in non-small cell lung cancer patients treated with anti-PD1 immunotherapy. Cancer Med 8(18):7669–7678. https://doi.org/10.1002/cam4.2632
Wang Z, Duan J, Wang G, Zhao J, Xu J, Han J, Zhao Z, Zhao J, Zhu B, Zhuo M, Sun J, Bai H, Wan R, Wang X, Fei K, Wang S, Zhao X, Zhang Y, Huang M, Huang D, Qi C, Gao C, Bai Y, Dong H, Xiong L, Tian Y, Wang D, Xu C, Wang W, Li J, Hu X, Cai S, Wang J (2019) Allele frequency-adjusted blood-based tumor mutational burden as a predictor of overall survival for patients with NSCLC treated With PD-(L)1 inhibitors. J thorac oncol : off publ Int Assoc Stud Lung Cancer. https://doi.org/10.1016/j.jtho.2019.12.001
Chae YK, Davis AA, Agte S, Pan A, Simon NI, Iams WT, Cruz MR, Tamragouri K, Rhee K, Mohindra N, Villaflor V, Park W, Lopes G, Giles FJ (2019) Clinical implications of circulating tumor DNA tumor mutational burden (ctDNA TMB) in non-small cell lung cancer. Oncologist 24(6):820–828. https://doi.org/10.1634/theoncologist.2018-0433
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours revised RECIST. Eur j cancer 45(2):228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Xie Z, Liu L, Lin X, Xie X, Gu Y, Liu M, Zhang J, Ouyang M, Lizaso A, Zhang H, Feng W, Li B, Han-Zhang H, Chen S, Li S, Zhong N, Liu H, Zhou C, Qin Y (2019) A multicenter analysis of genomic profiles and PD-L1 expression of primary lymphoepithelioma-like carcinoma of the lung. Modern pathol : off j U S Canad Acad Pathol. https://doi.org/10.1038/s41379-019-0391-9
Bantis LE, Nakas CT, Reiser B (2014) Construction of confidence regions in the ROC space after the estimation of the optimal Youden index-based cut-off point. Biometrics 70(1):212–223. https://doi.org/10.1111/biom.12107
Stenzinger A, Endris V, Budczies J, Merkelbach-Bruse S, Kazdal D, Dietmaier W, Pfarr N, Siebolts U, Hummel M, Herold S, Andreas J, Zoche M, Togel L, Rempel E, Maas J, Merino D, Stewart M, Zaoui K, Schlesner M, Glimm H, Frohling S, Allen J, Horst D, Baretton G, Wickenhauser C, Tiemann M, Evert M, Moch H, Kirchner T, Buttner R, Schirmacher P, Jung A, Haller F, Weichert W, Dietel M (2020) Harmonization and standardization of panel-based tumor mutational burden (TMB) measurement: real-world results and recommendations of the QuIP study. J thorac oncol : off publ Int Assoc Stud Lung Cancer. https://doi.org/10.1016/j.jtho.2020.01.023
Wu HX, Wang ZX, Zhao Q, Wang F, Xu RH (2019) Designing gene panels for tumor mutational burden estimation: the need to shift from “correlation” to “accuracy.” J Immun Cancer 7(1):206. https://doi.org/10.1186/s40425-019-0681-2
Kazdal D, Endris V, Allgauer M, Kriegsmann M, Leichsenring J, Volckmar AL, Harms A, Kirchner M, Kriegsmann K, Neumann O, Brandt R, Talla SB, Rempel E, Ploeger C, von Winterfeld M, Christopoulos P, Merino DM, Stewart M, Allen J, Bischoff H, Meister M, Muley T, Herth F, Penzel R, Warth A, Winter H, Frohling S, Peters S, Swanton C, Thomas M, Schirmacher P, Budczies J, Stenzinger A (2019) Spatial and temporal heterogeneity of panel-based tumor mutational burden in pulmonary adenocarcinoma: separating biology from technical artifacts. J thorac oncol : off publ Int Assoc Stud Lung Cancer 14(11):1935–1947. https://doi.org/10.1016/j.jtho.2019.07.006
Buttner R, Longshore JW, Lopez-Rios F, Merkelbach-Bruse S, Normanno N, Rouleau E, Penault-Llorca F (2019) Implementing TMB measurement in clinical practice: considerations on assay requirements. ESMO open 4(1):e000442. https://doi.org/10.1136/esmoopen-2018-000442
Budczies JAM, Litchfield K et al (2019) Optimizing panel-based tumor mutational burden (TMB) measurement. Ann oncol: off j Eur Soc Med Oncol 30(9):1496–1506
Jamal-Hanjani M, Wilson GA, McGranahan N, Birkbak NJ, Watkins TBK, Veeriah S, Shafi S, Johnson DH, Mitter R, Rosenthal R, Salm M, Horswell S, Escudero M, Matthews N, Rowan A, Chambers T, Moore DA, Turajlic S, Xu H, Lee SM, Forster MD, Ahmad T, Hiley CT, Abbosh C, Falzon M, Borg E, Marafioti T, Lawrence D, Hayward M, Kolvekar S, Panagiotopoulos N, Janes SM, Thakrar R, Ahmed A, Blackhall F, Summers Y, Shah R, Joseph L, Quinn AM, Crosbie PA, Naidu B, Middleton G, Langman G, Trotter S, Nicolson M, Remmen H, Kerr K, Chetty M, Gomersall L, Fennell DA, Nakas A, Rathinam S, Anand G, Khan S, Russell P, Ezhil V, Ismail B, Irvin-Sellers M, Prakash V, Lester JF, Kornaszewska M, Attanoos R, Adams H, Davies H, Dentro S, Taniere P, O’Sullivan B, Lowe HL, Hartley JA, Iles N, Bell H, Ngai Y, Shaw JA, Herrero J, Szallasi Z, Schwarz RF, Stewart A, Quezada SA, Le Quesne J, Van Loo P, Dive C, Hackshaw A, Swanton C (2017) Tracking the evolution of non-small-cell lung cancer. N Engl J Med 376(22):2109–2121. https://doi.org/10.1056/NEJMoa1616288
Abbosh C, Birkbak NJ, Wilson GA, Jamal-Hanjani M, Constantin T, Salari R, Le Quesne J, Moore DA, Veeriah S, Rosenthal R, Marafioti T, Kirkizlar E, Watkins TBK, McGranahan N, Ward S, Martinson L, Riley J, Fraioli F, Al Bakir M, Gronroos E, Zambrana F, Endozo R, Bi WL, Fennessy FM, Sponer N, Johnson D, Laycock J, Shafi S, Czyzewska-Khan J, Rowan A, Chambers T, Matthews N, Turajlic S, Hiley C, Lee SM, Forster MD, Ahmad T, Falzon M, Borg E, Lawrence D, Hayward M, Kolvekar S, Panagiotopoulos N, Janes SM, Thakrar R, Ahmed A, Blackhall F, Summers Y, Hafez D, Naik A, Ganguly A, Kareht S, Shah R, Joseph L, Marie Quinn A, Crosbie PA, Naidu B, Middleton G, Langman G, Trotter S, Nicolson M, Remmen H, Kerr K, Chetty M, Gomersall L, Fennell DA, Nakas A, Rathinam S, Anand G, Khan S, Russell P, Ezhil V, Ismail B, Irvin-Sellers M, Prakash V, Lester JF, Kornaszewska M, Attanoos R, Adams H, Davies H, Oukrif D, Akarca AU, Hartley JA, Lowe HL, Lock S, Iles N, Bell H, Ngai Y, Elgar G, Szallasi Z, Schwarz RF, Herrero J, Stewart A, Quezada SA, Peggs KS, Van Loo P, Dive C, Lin CJ, Rabinowitz M, Aerts H, Hackshaw A, Shaw JA, Zimmermann BG, Swanton C (2017) Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature 545(7655):446–451. https://doi.org/10.1038/nature22364
Zhang Y, Chang L, Yang Y, Fang W, Guan Y, Wu A, Hong S, Zhou H, Chen G, Chen X, Zhao S, Zheng Q, Pan H, Zhang L, Long H, Yang H, Wang X, Wen Z, Wang J, Yang H, Xia X, Zhao Y, Hou X, Ma Y, Zhou T, Zhang Z, Zhan J, Huang Y, Zhao H, Zhou N, Yi X, Zhang L (2019) The correlations of tumor mutational burden among single-region tissue, multi-region tissues and blood in non-small cell lung cancer. J Immun Cancer 7(1):98. https://doi.org/10.1186/s40425-019-0581-5
Yu H, Chen Z, Ballman KV, Watson MA, Govindan R, Lanc I, Beer DG, Bueno R, Chirieac LR, Chui MH, Chen G, Franklin WA, Gandara DR, Genova C, Brovsky KA, Joshi MM, Merrick DT, Richards WG, Rivard CJ, Harpole DH, Tsao MS, van Bokhoven A, Shepherd FA, Hirsch FR (2019) Correlation of PD-L1 expression with tumor mutation burden and gene signatures for prognosis in early-stage squamous cell lung carcinoma. J thorac oncol : off publ Int Assoc Stud Lung Cancer 14(1):25–36. https://doi.org/10.1016/j.jtho.2018.09.006
Singal G, Miller PG, Agarwala V, Li G, Kaushik G, Backenroth D, Gossai A, Frampton GM, Torres AZ, Lehnert EM, Bourque D, O’Connell C, Bowser B, Caron T, Baydur E, Seidl-Rathkopf K, Ivanov I, Alpha-Cobb G, Guria A, He J, Frank S, Nunnally AC, Bailey M, Jaskiw A, Feuchtbaum D, Nussbaum N, Abernethy AP, Miller VA (2019) Association of patient characteristics and tumor genomics with clinical outcomes among patients with non-small cell lung cancer using a clinicogenomic database. JAMA 321(14):1391–1399. https://doi.org/10.1001/jama.2019.3241
Marcin Kowanetz WZ, Shames D, Cummings C, Rizvi N, Spira A, Frampton G, Leveque V, Flynn S, Mocci S, Shankar G, Funke R, Ballinger M, Waterkamp D, Chen D, Sandler A, Hampton G, Amler L, Hegde P, Hellmann M (2017) Tumor mutation burden (TMB) is associated with improved efficacy of atezolizumab in 1L and 2L+ NSCLC patients. J Thorac Oncol 12(1):S321-322
Singal GMP, Agarwala V et al (2017) Analyzing biomarkers of cancer immunotherapy (CIT) response using a real-world clinicogenomic database. Ann oncol: off j Eur Soc Med Oncol 28:v403–v427
Kowanetz MZW, Shames D et al (2017) OA20.01 Tumor Mutation burden (TMB) is associated with improved efficacy of atezolizumab in 1L and 2L+ NSCLC patients. J thorac oncol: off publ Int Assoc Stud Lung Cancer 12:S321–S322
Funding
This work was supported by the grants from the National Key R&D Program of China (grant number: 2016YFC1303800) and the National Natural Science Foundation of China (grant number: 81972179).
Author information
Authors and Affiliations
Contributions
Xi Chen contributed to conceptualization, methodology, supervision, project administration, writing—original draft; Liangjie Fang contributed to methodology, data curation, resources; Yanping Zhu contributed to methodology, data curation, resources; Zhang Bao contributed to methodology, data curation, resources; Qing Wang contributed to methodology and resources; Rong Liu contributed to methodology and resources; Wenjia Sun contributed to methodology and resources; Haiwei Du contributed to writing—original draft, data curation; Jing Lin contributed to formal analysis, data curation, writing—review and editing; Bing Yu contributed to data curation, writing—review and editing; Songan Chen contributed to data curation, writing—review and editing; Jianya Zhou contributed to conceptualization, methodology, supervision, project administration, writing—review and editing; Jianying Zhou contributed to conceptualization, methodology, supervision, project administration.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics approval
This study was approved by the Ethics Committee of the First Affiliated Hospital of College of Medicine of Zhejiang University.
Consent to participate
Informed consent was obtained from each patient for the use of their peripheral blood and tumor tissue samples.
Consent for publication
Obtained.
Availability of data and material
Data are not publicly available.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, X., Fang, L., Zhu, Y. et al. Blood tumor mutation burden can predict the clinical response to immune checkpoint inhibitors in advanced non-small cell lung cancer patients. Cancer Immunol Immunother 70, 3513–3524 (2021). https://doi.org/10.1007/s00262-021-02943-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-021-02943-2