Abstract
Purpose
Obesity and insulin resistance appear to worsen prognosis of breast cancer patients. We conducted a feasibility study to test a 5:2 fasting regime in breast cancer patients undergoing radiotherapy. The intervention was rated as beneficial if it would be able to reduce fat mass while significantly improving insulin sensitivity.
Methods
A total of 13 non-metastatic breast cancer patients were recruited and instructed to completely abstain from food on two non-consecutive days (minimum 24 h) per week during radiotherapy. Body composition was measured weekly by bioimpedance analysis. Blood parameters were assessed before and at the end of radiotherapy. The product of triglycerides and glucose was used as a proxy for insulin sensitivity. A control group on an unspecified standard diet was assigned by propensity score matching.
Results
A total of twelve patients completed the study. Three patients reported side effects during fasting which were mild (grade 1). Two patients reported feeling bad while fasting, whereas five had a generally good or very good feeling. The fasting group experienced an average decrease of approximately 200 g body mass (p < 0.0001), 200 g (p = 0.002) fat mass and 100 g muscle mass (p = 0.047) per week, resulting in absolute reductions of 2.45 ± 1.19 kg body mass, 1.5 ± 1.6 kg fat mass and 0.7 ± 0.4 kg muscle mass. There was no improvement in insulin sensitivity and other markers of metabolic health except for gamma-glutamyltransferase which decreased by -7 ± 8 U/l. There was also no indication that 5:2 fasting protected against acute skin toxicity.
Conclusions
5:2 fasting is safe and feasible for breast cancer patients during radiotherapy and suitable to significantly reduce fat mass, but beneficial metabolic effects could not be confirmed. To improve these results, future studies could combine 5:2 fasting with carbohydrate restriction, increased protein intake and/or exercise.
Trial registration
Registered on ClinicalTrials.gov under NCT05861362 on May 12, 2023 (retrospectively registered; https://clinicaltrials.gov/ct2/show/NCT05861362).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Female breast cancer (BC) is now the most commonly diagnosed non-cutaneous cancer worldwide, with an estimated 2.3 million global new cases in 2020, and it is the fifth leading cause of cancer mortality worldwide [1]. In Germany, according to 2018 incidence rates, approximately one in eight women is expected to develop BC within her lifetime [2], which poses a relevant economic burden for the health insurance system [3].
The annual incidence of estrogen receptor-positive BC cases is increasing within western countries, a development which is partly explained by the current obesity epidemic [1]. Adipose tissue becomes the dominant site of estrogen biosynthesis in postmenopausal women via the enzyme aromatase, partially explaining this relationship [4]. Aromatase is also localized within mammary adipose tissue and in BC tissue itself [5], so that both systemic and local estrogen levels may contribute to estrogen receptor-positive tumorigenesis. In addition, there are more general mechanisms explaining how obesity contributes to BC risk. Adipose tissue is also the source of pro-inflammatory adipokines which can promote tumorigenesis at various sites including the breast [6]. Increased levels of pro-inflammatory adipokines also promote insulin resistance in peripheral tissues, leading to an elevation of blood glucose and insulin concentrations and a decrease in insulin-like growth factor-1 (IGF-1) binding proteins, resulting in higher bioavailable IGF-1 concentrations. Glucose, insulin and IGF-1 are potent stimulants of BC cell proliferation in vitro and in vivo [7, 8]. In the human estrogen receptor-positive BC cell line MCF-7 it has been shown that insulin reduces sensitivity to the antiestrogens tamoxifen and nafoxidine [9], primes the cells’ response to subsequent estradiol treatment [10] and synergizes with estrogen to induce cell cycle progression from the G1 to the S phase [11]. In non-diabetic BC patients, higher pretreatment insulin concentrations are associated with worse progression-free survival independently of menopausal status, disease stage, hormone receptor status, and human epidermal growth factor receptor 2 and Ki67 expression [12]. A review of the literature on obesity and BC outcomes by Azrad and Demark-Wahnefried showed that obesity and related biomarkers such as serum insulin levels at the time of BC diagnosis are predictors of a poor prognosis [13].
This poses the question whether nutritional interventions in BC patients could reduce obesity, improve insulin sensitivity and in this way reduce the risk for BC recurrence or progression. Unfortunately, dietary advice given to cancer patients is inconsistent, and identifying the appropriate nutritional intervention is difficult [14]. We have previously shown that both a ketogenic diet (KD) [15] and a Paleolithic diet combined with daily physical activity [16] were able to induce significant weight loss during radiotherapy (RT) of BC patients, primarily through a reduction of fat mass. Concurrently, concentrations of several metabolic biomarkers such as triglycerides, IGF-1, T3 hormone and gamma-glutamyl transpeptidase (GGT), an indicator of non-alcoholic fatty liver disease (NAFLD), improved through these interventions [16, 17]. Another dietary strategy to promote weight loss and improve metabolic health is calorie restriction, either in the form of chronic calorie restriction (CCR) or intermittent fasting (IF), which alternates periods of no or minimal caloric intake with ad libitum eating. IF has received increasing interest within BC research due to its putatively beneficial effects when used in combination with chemotherapy [18,19,20,21]. In our opinion, RT provides a great opportunity to introduce a dietary change with the aim of improving metabolic health, since during this period patients can be regularly monitored and motivated. Given our previous positive experiences with both KD and Paleolithic diet regimens, we aimed to undertake a new feasibility study to test the effects of a 5:2 IF regime in BC patients undergoing RT. 5:2 fasting consists of two (usually separated) fasting days and five days of normal eating per week. The reason for studying this particular regime was that IF on two days per weeks was shown to elicit greater reductions in body mass (BM) and fat mass (FM) than CCR in BC patients undergoing chemotherapy [20]. Our hypothesis was that this intervention might be difficult to sustain, but result in significant FM loss and improvements in insulin sensitivity.
2 Materials and methods
2.1 Study population
Eligible patients for this feasibility study were non-metastasized breast cancer patients referred to our clinic for curative adjuvant radiotherapy after breast conservation surgery. Exclusion criteria consisted of metallic body parts that would interfere with bioelectrical impedance analysis (BIA) measurements, difficulties with understanding the aims of the study and age older than 75 years. Diabetes or thyroid disorders were no exclusion criterion. Patients were enrolled from June 1, 2022 to October 17, 2022. The study protocol was similar to that of our KETOCOMP study for which ethical approval was granted by the Bavarian Medical Association (proposal number 15025) and which was registered on 08/06/2015 under ClinicalTrials.gov (Identifier: NCT02516501) [22]. Prior to enrollment, all patients provided written informed consent to participate.
Control (CTRL) patients were taken from the KETOCOMP study control group of BC patients who consumed their standard diet. For each patient in the 5:2 fasting (FAST) group who finished the study (N = 12), a CTRL group patient was assigned through nearest-neighbor matching on the propensity score [23, 24]. The propensity score was estimated via a logistic regression of the treatment group variable (0 = CTRL; 1 = FAST) on a constant term and baseline measurements of age, body mass index (BMI), fat mass (FM), exercise (yes/no) and radiotherapy fractionation type (hypofractionation/normofractionation). The choice to use these covariates was their putative causal influence on body composition and metabolic blood parameters.
2.2 Measurements and intervention
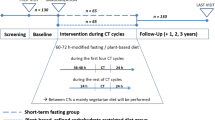
The procedures for this pilot study closely followed those of the KETOCOMP study [15, 22, 25]. For initial (baseline) measurements, patients presented fasted and with an empty bladder on the same morning they received their radiotherapy planning computed tomography. This was approximately one week prior to the start of radiotherapy. The following baseline measurements were performed:
-
Body mass (BM) estimation and BIA on a calibrated seca 515/514 medical Body Composition Analyzer (mBCA; seca Deutschland, Hamburg, Germany) estimating fat free mass (FFM), skeletal muscle mass (SMM), total body water (TBW), extracellular water (ECW) and phase angle at 50 kHz (PA); from these, FM was derived as FM = BM − FFM and intracellular water (ICW) as ICW = TBW − ECW
-
The validated EORTC QLC-C30 questionnaire version 3.0
-
Blood draw with subsequent analysis in the hospital laboratory (including glucose, lactate, lactate dehydrogenase, cholesterol, triglycerides, liver enzymes, insulin, IGF-1, free T3).
BIA and BM estimation were repeated weekly during radiotherapy. These measurements were performed in the morning (mostly between 7:30 and 9:30 a.m.) prior to irradiation. For standardization, patients were advised to fast prior to BIA and keep their bladder empty. After each BIA measurement capillary glucose concentrations were checked by obtaining blood through finger pricks that was analyzed with the FreeStyle Precision device (Abbott Diabetes Care Ltd., Range Road, Witney, UK); therefore, we obtained at least one glucose measurement per week. In addition, laboratory blood analysis and completion of the quality of life questionnaire were repeated in the final week of radiotherapy.
The FAST intervention consisted of two nonconsecutive days of fasting per week that could be chosen freely according to the patient’s weekly schedule. The minimum amount of time to count as a fasting day was 24 h, but patients were advised to aim for a complete fasting day including the nights before and after that day. On a fasting day, only water and unsweetened tea or coffee was allowed. However, given that fat is the macronutrient interfering the least with the metabolic adaptions to fasting, while carbohydrates disturb the most [26], patients were allowed to consume small amounts of bone broth/meat broth, coconut oil, butter/ghee or heavy cream as an “emergency plan”, i.e., in case that they felt they needed some energy-containing foods to complete an initiated fasting day.
Patients were counseled to start their first fasting day at least two days prior to the first irradiation. Compliance was monitored at each weekly measurement appointment. At the final measurement, patients in the FAST group also received a self-designed, non-validated questionnaire to inquire about their subjective feelings and attitude towards the intervention.
2.3 Outcomes of interest
The primary study outcome was the feasibility of the FAST intervention and its effects on longitudinal body composition changes from baseline until the final week of radiotherapy. The intervention was rated as feasible if the dropout rate was < 30%. Secondary endpoints were absolute changes in metabolic parameters, hormones, and overall quality of life scores in the FAST group. As a surrogate marker for insulin resistance, the trigylceride-glucose (TyG) index was calculated according to [27]
Overall, the intervention was rated as beneficial if it would be able to reduce FM while significantly reducing the TyG index.
2.4 Statistical analysis
The longitudinal body composition data of the 24 patients from the FAST and CTRL group were analyzed using linear mixed effects models with the intercept and slope of the variable \(t\) (time since start of RT) as random effects varying by the individual patient. Let \({y}_{ij}\), \(i=1,\dots ,{n}_{j}\), denote the \(i\) th measurement on patient \(j=1,\dots ,24\) at time \({t}_{i}\) during RT. Previously we had shown that age and initial BMI significantly influenced time trends in FFM, SMM, TBW and ICW or BW, respectively [15]. Because we now had matched both groups with respect to these confounders, we only constructed simple models predicting an individual body composition measurement \({y}_{ij}\) based on time, group (0 = CTRL; 1 = FAST), their interaction and the corresponding baseline body composition measure \({y}_{1j}\):
The average time trends of patients in the CTRL and FAST group are therefore given by \({\beta }_{1}\) and \({\beta }_{1}+{\beta }_{3}\), respectively.
Differences between continuous and categorical variables were assessed using the Mann–Whitney-Wilcoxon and Fisher's exact test, respectively. We did not correct p-values for multiple tests [28], but decided to only consider p-values < 0.01 indicating “significant” findings. This threshold was chosen based on the conversion between p-values and minimum Bayes factors [29] which measure the strength of evidence between two competing hypotheses. In exploratory analyses, a p-value of 0.01 corresponds to a minimum Bayes factor of 1/6.5, providing moderate-strong evidence against the null hypothesis [29]. All analyses were carried out in R, version 4.2.2 with the software packages “lme4” for linear mixed effects modeling and “MatchIt” [23] for nearest-neighbor matching.
3 Results
3.1 General results
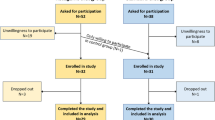
A flowchart displaying the recruitment process of the study population is given in Fig. 1. One patient in the FAST group ended the study after experiencing headache during the first fasting day and failing to conduct a second fasting day due to strong food cravings. Because 12/13 patients (92.3%) were able to finish the study with fulfilling the required fasting periods in each week, the intervention of 5:2 fasting during radiotherapy was judged as feasible.
The baseline characteristics of the study population used for analysis are given in Table 1. After propensity score matching, there were no significant differences in any of the listed parameters, although a higher number of smokers in the CTRL group compared to the FAST group almost reached the threshold of statistical significance (p = 0.018).
All patients completed RT as prescribed. Three patients had received hypofractionated RT with less than 20 fractions (most frequently 16 × 2.5 Gy/ 16 × 3 Gy single integrated boost) while 9 had received normofractionated RT (most frequently 28 × 1.8 Gy/ 28 × 2.15 Gy single integrated boost).
3.2 Feasibility of the intervention
Table 2 shows results concerning the feasibility of the FAST intervention. The number of fasts ranged from 8 to 24. Three patients reported side effects during fasting which were mild (grade 1). Two patients (17%) reported feeling bad while fasting, whereas five (42%) had a generally good or very good feeling. Four patients (33%) rated the practicability of fasting as difficult, while the majority (58%) found it easy or very easy. However, most patients modified some of their fasts according to our option to abbreviate the fasting duration from one full day (including the prior and following night) to only parts of two days and one full night in-between with a minimum fasting duration of 24 h. Two patients also took the option to add some cream into their coffee while fasting. Most patients took a vitamin D supplement that was prescribed after having measured insufficient vitamin D levels (< 40 ng/ml) [30].
3.3 Effects on body composition
Absolute changes in body composition parameters are given in Table 3. Compared to the CTRL group, the FAST group experienced a significant reduction in all body composition parameters, except for phase angle which remained approximately stable in both groups.
The individual changes in BM, FM and SMM are plotted in Fig. 2. Patients in the FAST group experienced decreases in BM and FM to varying extents, but best visible is a clear trend of gradual SMM loss. The results of the mixed effects model fits to the body composition data are given in Table 4. No significant time trends were observed for the CTRL group. In contrast, the FAST group experienced a significant drop of BM of approximately 400 g/week to which FM contributed roughly 200 g/week. FFM was not significantly affected and there were no significant trends in phase angle or water distribution in the FAST group.
3.4 Effects on metabolic blood parameters and hormones
Table 3 shows the changes in blood parameters for both groups from baseline until study end. A between-group comparison showed that there were no significant differences in any blood parameter changes between groups. All blood parameter changes that occurred between the baseline and final measurement within the FAST group were also not statistically significant (paired Wilcoxon test, all p > 0.01) except for GGT (p = 0.0099).
3.5 Effects on overall quality of life
Overall quality of life (items 29 and 30 in the EORTC QLC-C30 questionnaire) remained stable between the baseline and final measurement, with no significant changes compared to the CTRL group (Table 3).
3.6 Effects on skin toxicity
Because in theory intermittent fasting may protect against chemotherapy as well as radiotherapy side effects [18,19,20,21, 31], we investigated early breast skin toxicity at the end of radiotherapy. Table 5 shows the skin toxicity experienced by the women at the end of radiotherapy according to treatment group. Only acute erythema was observed, but there was no significant difference in the severity of skin toxicity among both groups (p = 0.291).
3.7 Comparison with a ketogenic diet
As an additional analysis, we matched the FAST group to a group of patients who had consumed a KD during RT within the KETOCOMP study [15, 17] using the same propensity score matching equation as for the CTRL group. As shown in Supplementary Table 1, matching resulted in a KD group with similar characteristics as the FAST group, with no significant difference in any of the baseline variables between groups. The absolute changes in body composition and blood parameters are given in Supplementary Table 2. There were no significant differences between the FAST and the KD group with respect to body composition changes and with respect to most blood parameter changes. However, compared with the FAST group, the KD group had significantly greater decreases in triglycerides, the TyG index and T3 concentrations.
4 Discussion
In this study, we had tested the feasibility and metabolic effects of a 5:2 IF intervention in BC patients concurrently receiving RT. To our knowledge, this is the first study to test such an intervention for cancer patients during RT. Our initial hypothesis was that a substantial proportion of women would drop out of the study as the literature indicated that both IF and CCR appear difficult to sustain in cancer patients. Champ and Klement identified poor completion and lack of reporting of results in the majority of studies assessing calorie restriction for cancer prevention or treatment [32]. They concluded that these findings may indicate poor compliance or excessive toxicity. However, we found that the greatest hurdle for implementation of the FAST intervention was the recruitment phase as 50% of eligible patients declined to participate. Of those 13 patients who were willing and motivated to take part in the study, 12 (92%) also completed the study. This finding is in line with high adherence rates to different IF regimes in studies lasting shorter than 3 months conducted in non-cancer patients [33]. Although our version of the 5:2 diet was stricter than the conventional version which usually allows the consumption of 500 kcal/d (2092 kJ) on fasting days for females [34], the option to abbreviate the fasting duration to a minimum of 24 h was probably helpful for achieving this high rate of adherence.
Although – as initially hypothesized – the intervention was successful in inducing significant BM and FM loss, SMM was also reduced significantly (Table 3). Compared to an unrestricted standard diet, women in the FAST group lost about 400 g of BM and 200 g of FM per week (Table 4), resulting in absolute reductions of 2.45 ± 1.19 kg BM and 1.5 ± 1.6 kg FM from baseline until study end. The gradual reduction in SMM was 0.10 ± 0.05 kg/week (p = 0.047), resulting in an absolute reduction of 0.7 ± 0.4 kg which was statistically significant compared to the CTRL group. We were not able to detect any significant differences in gradual or absolute body composition changes with respect to a KD group which we had also matched to the FAST group using propensity scores. This indicates that the FAST and KD interventions exerted similar effects on body composition and could both be options when the goal is to reduce FM over the short-term.
However, compared to the FAST intervention, the KD intervention was superior in reducing triglycerides, T3 levels and the TyG index, a marker of insulin resistance (Supplementary Table 2). In fact, our initial hypothesis that 5:2 fasting would improve insulin sensitivity was not confirmed, and therefore the intervention was not rated as beneficial. The TyG index has been proposed as a surrogate marker of insulin resistance by Simental-Mendía and colleagues [27], which was confirmed by a meta-analysis, although exact diagnostic cutoff values remain uncertain [35]. According to Son et al., the TyG index is also superior to the homeostasis model assessment of insulin resistance (HOMA-IR) which utilizes measurements of insulin and glucose [36]. Recent studies found a dose–response relationship between the TyG index and BC risk [37] as well as cardiovascular disease risk among cancer survivors [38]. Failure of the FAST intervention to improve this surrogate marker may either indicate that 5 days of ad libitum eating have offset the expected positive effects of fasting on insulin resistance or that the duration of the intervention was too short. The former possibility appears more likely since the short-term KD significantly reduced triglycerides and the TyG index, pointing out overall diet quality as an important determinant of metabolic health. Other data has shown that two weeks of CCR allow for a significant reduction of fasting triglyceride concentrations and that carbohydrate restriction has additional beneficial effects on hepatic production of triglycerides [39].
The lack of improvement in triglycerides, cholesterol, insulin and glucose levels is consistent with the overall failure of other short-term (≤ 3 months) IF regimes to improve these parameters [40]. However, the meta-analysis of Yin et al. [40] provided evidence that such interventions could reduce concentrations of the liver enzymes ALT and AST, indicative of beneficial effects against NAFLD; the effects on GGT, however, were not assessed in this meta-analysis. In our study, ALT and AST did not change in the FAST group, but there was a significant reduction of GGT concentration which could also indicate efficacy against NAFLD.
Because interest in IF in oncology has mostly been raised in association with its putative protection against chemotherapy side effects [18,19,20,21], we also evaluated the maximum skin toxicity at the end of radiotherapy which was restricted to acute erythema, but no desquamation. However, no significant differences between the FAST and control group could be seen. As shown by Twardella et al. [41], acute skin toxicity is mainly influenced by BMI which correlates with breast size. We believe that the average BMI reduction in the FAST group of < 1 kg/m2 (Table 3) was too small to translate into any clinical benefit regarding acute skin toxicity, and that a more significant BMI reduction in the months prior to RT would be a better option for breast cancer patients to reduce their risk of skin toxicity.
Some more limitations of this feasibility study need to be pointed out: First, the sample size (N = 12) is one of the main limitations because it limits statistical power. Secondly, we limited the duration of the study to the period during which patients received RT and did not evaluate long-term effects. Thirdly, we point out that the 5:2 IF regime applied in this study is only one variety of calorie restriction. The most frequently studied variety of calorie restriction is CCR with a chronic 20–50% reduction of energy intake compared to ad libitum feeding without inducing malnutrition. However, as we pointed out in the introduction, the reason to study IF instead of CCR was its greater potential for reducing body fat mass. Fourthly, women were free to choose their diets according to their preferred eating habits (except for fasting days in the FAST group). Thus, the role of energy intake and dietary macronutrient composition remain elusive. However, despite not controlling for these variables we obtained robust results that are likely to translate into other “real world” settings, i.e., have a high external validity. Fifthly, our inclusion criteria were rather broad since we did not focus on a defined hormone receptor status or BMI range. The reason for this was that we were mainly interested in the general feasibility and broader metabolic effects within a “real world” setting. For example, not only patients with obesity may decide to undergo an intermittent fasting regime, but also those with normal BMI, e.g., with the goal to improve their metabolic health. However, as we did not find improvements of most metabolic health parameters tested, we see little benefits of 5:2 fasting for women who are already lean and have a normal weight. Finally, we did not directly study putative anti-tumor effects of the FAST intervention which is difficult to do in such a patient cohort with generally good prognosis after RT, as it would require many years of follow-up and a larger sample size. Hence, no information on the effects of IF during RT on tumor control and overall survival is available. Preclinical data suggest that calorie restriction is effective in delaying the growth of breast cancer. For example, a meta-analysis by Dirx et al. [42] from 2003 provided convincing evidence that CCR is able to lower the risk for spontaneous mammary tumors in mice. In a murine 4T1 breast cancer model, 20% CCR was more effective in inhibiting tumor growth than a caloric cycling regime using a plant-based fasting mimicking diet in which calories were reduced for 4 days followed by 10 days ad libitum eating [43]. While these data appear promising, the translation towards human patients remains uncertain since it is known that fasting for 1 day in the mouse is roughly equivalent to a one-week water-only fast in a human [44]. Specifically, a one-week water only fast would result in significant reductions of IGF-1 and elevations of ketone body concentrations. While we did not systematically test ketone body concentrations in our patients, another study found that in females doing 5:2 fasting for one week, β-hydroxybutyrate concentrations in the evening and next morning after a fasting day can rise to levels > 1 mmol/l [45].
5 Conclusions
In conclusion, a 5:2 fasting diet was feasible as a complementary treatment of breast cancer patients undergoing RT, and led to significant reductions of BM, FM, FFM and SMM. However, the intervention failed to significantly improve markers of insulin resistance and most other metabolic health indicators and hence was not rated as beneficial. Through a comparison with a KD we were able to show that reducing carbohydrate intake without limiting calories is similarly efficient to the 5:2 fasting intervention in reducing FM but appears to have a greater potential for metabolic improvements. As recent data have revealed the importance of body composition—i.e. increased SMM and decreased FM—with outcomes after treatment for breast cancer, the loss of SMM within the context of a non-improvement of insulin sensitivity seen in this study is concerning. Future efforts may wish to address this through an increase in protein intake and/or exercise programs utilizing resistance training to optimize FM loss while preserving SMM. This may also impact insulin sensitivity [46].
Availability of data and materials
All data used for analysis can be requested from the corresponding author.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. https://doi.org/10.3322/caac.21660.
Robert Koch Institut. Krebs in Deutschland für 2017/2018. 13th edition. Berlin: Robert Koch Institute; 2021. URL: https://www.krebsdaten.de/Krebs/DE/Content/Publikationen/Krebs_in_Deutschland/krebs_in_deutschland_inhalt.html.
Kreis K, Plöthner M, Schmidt T, Seufert R, Schreeb K, Jahndel V, et al. Healthcare costs associated with breast cancer in Germany: a claims data analysis. Eur J Heal Econ. 2020;21:451–64. https://doi.org/10.1007/s10198-019-01148-w.
Cleary MP, Grossmann ME. Minireview: obesity and breast cancer: the estrogen connection. Endocrinology. 2009;150:2537–42. https://doi.org/10.1210/en.2009-0070.
Miller WR. Aromatase and the breast: regulation and clinical aspects. Maturitas. 2006;54:335–41. https://doi.org/10.1016/j.maturitas.2006.04.020.
Christodoulatos GS, Spyrou N, Kadillari J, Psallida S, Dalamaga M. The role of adipokines in breast cancer: current evidence and perspectives. Curr Obes Rep. 2019;8:413–33. https://doi.org/10.1007/s13679-019-00364-y.
Gallagher EJ, LeRoith D. Minireview: IGF, insulin, and cancer. Endocrinology. 2011;152:2546–51. https://doi.org/10.1210/en.2011-0231.
Klement RJ, Fink MK. Dietary and pharmacological modification of the insulin/IGF-1 system: exploiting the full repertoire against cancer. Oncogenesis. 2016;5: e193. https://doi.org/10.1038/oncsis.2016.2.
Butler WB, Kelsey WH, Goran N. Effects of serum and insulin on the sensitivity of the human breast cancer cell line MCF-7 to estrogen and antiestrogens. Cancer Res. 1981;41:82–8.
Wairagu PM, Phan ANH, Kim MK, Han J, Kim HW, Choi JW, et al. Insulin priming effect on estradiol-induced breast cancer metabolism and growth. Cancer Biol Ther. 2015;16:484–92. https://doi.org/10.1080/15384047.2015.1016660.
Mawson A, Lai A, Carroll JS, Sergio CM, Mitchell CJ, Sarcevic B. Estrogen and insulin/IGF-1 cooperatively stimulate cell cycle progression in MCF-7 breast cancer cells through differential regulation of c-Myc and cyclin D1. Mol Cell Endocrinol. 2005;229:161–73. https://doi.org/10.1016/j.mce.2004.08.002.
Ferroni P, Riondino S, Laudisi A, Portarena I, Formica V, Alessandroni J, et al. Pretreatment insulin levels as a prognostic factor for breast cancer progression. Oncologist. 2016;21:1041–9.
Azrad M, Demark-Wahnefried W. The association between adiposity and breast cancer recurrence and survival: A review of the recent literature. Curr Nutr Rep. 2014;3:9–15.
Champ CE, Mishra MV, Showalter TN, Ohri N, Dicker AP, Simone NL. Dietary recommendations during and after cancer treatment: consistently inconsistent? Nutr Cancer. 2013;65:430–9. https://doi.org/10.1080/01635581.2013.757629.
Klement RJ, Champ CE, Kämmerer U, Koebrunner PS, Krage K, Schäfer G, et al. Impact of a ketogenic diet intervention during radiotherapy on body composition: III—final results of the KETOCOMP study for breast cancer patients. Breast Cancer Res. 2020;22:94. https://doi.org/10.1186/s13058-020-01331-5.
Klement RJ, Koebrunner PS, Krage K, Weigel MM, Sweeney RA. Short-term effects of a Paleolithic lifestyle intervention in breast cancer patients undergoing radiotherapy: a pilot and feasibility study. Med Oncol. 2021;38:1. https://doi.org/10.1007/s12032-020-01443-0.
Klement RJ, Weigel MM, Sweeney RA. A ketogenic diet consumed during radiotherapy improves several aspects of quality of life and metabolic health in women with breast cancer. Clin Nutr. 2021;40:4267. https://doi.org/10.1016/j.clnu.2021.01.023.
de Groot S, Vreeswijk MP, Welters MJ, Gravesteijn G, Boei JJ, Jochems A, et al. The effects of short-term fasting on tolerance to (neo) adjuvant chemotherapy in HER2-negative breast cancer patients: a randomized pilot study. BMC Cancer. 2015;15:652. https://doi.org/10.1186/s12885-015-1663-5.
Bauersfeld SP, Kessler CS, Wischnewsky M, Jaensch A, Steckhan N, Stange R, et al. The effects of short-term fasting on quality of life and tolerance to chemotherapy in patients with breast and ovarian cancer: a randomized cross-over pilot study. BMC Cancer. 2018;18:476. https://doi.org/10.1186/s12885-015-1663-5.
Harvie M, Pegington M, Howell SJ, Bundred N, Foden P, Adams J, et al. Randomised controlled trial of intermittent vs continuous energy restriction during chemotherapy for early breast cancer. Br J Cancer. 2021;126:1157–67. https://doi.org/10.1038/s41416-021-01650-0.
Omar EM, Omran GA, Mustafa MF, El-Khodary NM. Intermittent fasting during adjuvant chemotherapy may promote differential stress resistance in breast cancer patients. J Egypt Natl Canc Inst. 2022;34:38. https://doi.org/10.1186/s43046-022-00141-4.
Klement RJ, Sweeney RA. Impact of a ketogenic diet intervention during radiotherapy on body composition: II. Protocol of a randomised phase I study (KETOCOMP). Clin Nutr ESPEN. 2016;12:1–6. https://doi.org/10.1016/j.clnesp.2015.11.001.
Ho DE, Imai K, King G, Stuart EA. MatchIt : Nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011;42:1–28.
Zhao Q-Y, Luo J-C, Su Y, Zhang Y-J, Tu G-W, Luo Z. Propensity score matching with R: conventional methods and new features. Ann Transl Med. 2021;9:812–812. https://doi.org/10.21037/atm-20-3998.
Klement RJ, Schäfer G, Sweeney RA. A ketogenic diet exerts beneficial effects on body composition of cancer patients during radiotherapy: an interim analysis of the KETOCOMP study. J Tradit Complement Med. 2020;10:180–7. https://doi.org/10.1016/j.jtcme.2019.03.007.
Klein S, Wolfe RR. Carbohydrate restriction regulates the adaptive response to fasting. Am J Physiol. 1992;262:E631–6.
Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6:299–304. https://doi.org/10.1089/met.2008.0034.
Perneger TV. What’s wrong with Bonferroni adjustments. BMJ. 1998;316:1236–8.
Held L, Ott M. On p-values and Bayes factors. Annu Rev Stat Appl. 2018;5:393–419.
Klement RJ, Koebrunner PS, Krage K, Sweeney RA. Low vitamin D status in a cancer patient population from Franconia. Germany Complement Med Res. 2021;28:300–7. https://doi.org/10.1159/000511993.
Klement RJ, Champ CE. Calories, carbohydrates, and cancer therapy with radiation: exploiting the five R’s through dietary manipulation. Cancer Metastasis Rev. 2014;33:217–29. https://doi.org/10.1007/s10555-014-9495-3.
Champ CE, Klement RJ. Assessing successful completion of calorie restriction studies for the prevention and treatment of cancer. Nutrition. 2020;78: 110829. https://doi.org/10.1016/j.nut.2020.110829.
Elortegui Pascual P, Rolands MR, Eldridge AL, Kassis A, Mainardi F, Lê K, et al. A meta-analysis comparing the effectiveness of alternate day fasting, the 5:2 diet, and time-restricted eating for weight loss. Obesity. 2022;31:9–21. https://doi.org/10.1002/oby.23568.
Scholtens EL, Krebs JD, Corley BT, Hall RM. Intermittent fasting 5:2 diet: What is the macronutrient and micronutrient intake and composition? Clin Nutr. 2020;39:3354–60. https://doi.org/10.1016/j.clnu.2020.02.022.
Sánchez-García A, Rodríguez-Gutiérrez R, Mancillas-Adame L, González-Nava V, Díaz González-Colmenero A, Solis RC, et al. Diagnostic accuracy of the triglyceride and glucose index for insulin resistance: a systematic review. Int J Endocrinol. 2020;2020:4678526. https://doi.org/10.1155/2020/4678526.
Son DH, Lee HS, Lee YJ, Lee JH, Han JH. Comparison of triglyceride-glucose index and HOMA-IR for predicting prevalence and incidence of metabolic syndrome. Nutr Metab Cardiovasc Dis. 2022;32:596–604. https://doi.org/10.1016/J.NUMECD.2021.11.017.
Panigoro SS, Sutandyo N, Witjaksono F, Siregar NC, Ramli R, Hariani R, et al. The association between triglyceride-glucose index as a marker of insulin resistance and the risk of breast cancer. Front Endocrinol (Lausanne). 2021;12: 745236. https://doi.org/10.3389/fendo.2021.745236.
Jung MH, Yi SW, An SJ, Yi JJ, Ihm SH, Han S, et al. Associations between the triglyceride-glucose index and cardiovascular disease in over 150,000 cancer survivors: a population-based cohort study. Cardiovasc Diabetol. 2022;21:52. https://doi.org/10.1186/s12933-022-01490-z.
Browning JD, Baker JA, Rogers T, Davis J, Satapati S, Burgess SC. Short-term weight loss and hepatic triglyceride reduction: Evidence of a metabolic advantage with dietary carbohydrate restriction. Am J Clin Nutr. 2011;93:1048–52. https://doi.org/10.3945/ajcn.110.007674.
Yin C, Li Z, Xiang Y, Peng H, Yang P, Yuan S, et al. Effect of intermittent fasting on non-alcoholic fatty liver disease: systematic review and meta-analysis. Front Nutr. 2021;8: 709683. https://doi.org/10.3389/fnut.2021.709683.
Twardella D, Popanda O, Helmbold I, Ebbeler R, Benner A, Von Fournier D, et al. Personal characteristics, therapy modalities and individual DNA repair capacity as predictive factors of acute skin toxicity in an unselected cohort of breast cancer patients receiving radiotherapy. Radiother Oncol. 2003;69:145–53. https://doi.org/10.1016/S0167-8140(03)00166-X.
Dirx MJM, Zeegers MPA, Dagnelie PC, Van Den Bogaard T, Van Den Brandt PA. Energy restriction and the risk of spontaneous mammary tumors in mice: a meta-analysis. Int J Cancer. 2003;106:766–70. https://doi.org/10.1002/ijc.11277.
Pomatto-Watson LCD, Bodogai M, Bosompra O, Kato J, Wong S, Carpenter M, et al. Daily caloric restriction limits tumor growth more effectively than caloric cycling regardless of dietary composition. Nat Commun. 2021;12:6201. https://doi.org/10.1038/s41467-021-26431-4.
Mahoney LB, Denny CA, Seyfried TN. Calorie restriction in C57BL/6J mice mimics therapeutic fasting in humans. Lipids Health Dis. 2006;5:13. https://doi.org/10.1186/1476-511X-5-13.
Cerniuc C, Fischer T, Baumeister A, Bordewick-Dell U. Impact of intermittent fasting (5:2) on ketone body production in healthy female subjects. Aktuelle Ernährungs Umschau. 2019;66:2–9. https://doi.org/10.4455/eu.2019.002.
Caan BJ, Cespedes Feliciano EM, Prado CM, Alexeeff S, Kroenke CH, Bradshaw P, et al. Association of muscle and adiposity measured by computed tomography with survival in patients with nonmetastatic breast cancer. JAMA Oncol. 2018;94612:1–7. https://doi.org/10.1001/jamaoncol.2018.0137.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection was performed by Rainer J. Klement, Jerome Figueroa, Sami Ok and Reinhart A. Sweeney. Rainer J. Klement analyzed the data and wrote the first draft of the manuscript. All other authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed in line with the principles of the Declaration of Helsinki. Approval of the measurement procedures was granted on June 26, 2015, by the Ethics Committee of the Bavarian Medical Association (proposal number 15025).
Consent for publication
Informed consent to undertake the study and have their data published was obtained from all individual participants included in the study.
Competing interests
RJK and CEC occasionally follow a ketogenic diet. CEC receives compensation for books and lectures pertaining to nutrition and exercise and is a scientific advisor for Simply Good Foods/Atkins and readout health. All other authors disclose that no competing interests, in particular no financial interests, related to this work do exist.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table S1.
Baseline characteristics of the fasting and ketogenic diet group. Supplementary Table S2. Changes in body composition and blood parameters.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Klement, R.J., Figueroa, J., Weigel, M. et al. Feasibility and metabolic effects of a 5:2 fasting intervention in women with breast cancer during radiotherapy. Holist Integ Oncol 2, 35 (2023). https://doi.org/10.1007/s44178-023-00058-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44178-023-00058-5