Abstract
Background
The prevention of subsequent cardiovascular disease (CVD) is an essential part of cancer survivorship care. We conducted the present study to investigate the association between the TyG index (a surrogate marker of insulin resistance) and the risk of cardiovascular disease (CVD) events in cancer survivors.
Methods
Adult cancer patients, who underwent routine health examinations during 2009–2010 and were survived for more than 5 years as of January 1, 2011, were followed for hospitalization of CVD (either ischemic heart disease, stroke, or heart failure) until December 2020. Cox model was used to calculate hazard ratios associated with baseline TyG index (loge [fasting triglyceride (mg) × fasting glucose (mg)/2]) for the CVD hospitalization.
Results
A total of 155,167 cancer survivors (mean age 59.9 ± 12.0 years, female 59.1%) were included in this study. A graded positive association was observed between TyG and CVD hospitalization. An 8% elevated risk for CVD hospitalization was observed for a TyG index of 8-8.4 (aHR 1.08 [95% CI 1.01–1.14]); 10% elevated risk for a TyG index of 8.5–8.9 (aHR 1.10 [95% CI 1.03–1.17]); 23% elevated risk for a TyG index of 9.0-9.4 (aHR 1.23 [95% CI 1.15–1.31]); 34% elevated risk for a TyG index of 9.5–9.9 (aHR 1.34 [95% CI 1.23–1.47]); and 55% elevated risk for a TyG index ≥ 10 compared to the reference group (TyG index < 8). Per 1-unit increase in the TyG index, a 16% increase in CVD hospitalization and a 45% increase in acute myocardial infarction hospitalization were demonstrated. Graded positive associations were evident for atherosclerotic CVD subtypes, such as ischemic heart disease, acute myocardial infarction, and ischemic stroke, but not for hemorrhagic stroke or heart failure.
Conclusions
The TyG index may serve as a simple surrogate marker for the risk stratification of future CVD events, particularly atherosclerotic subtypes, in cancer survivors.
Similar content being viewed by others
Background
Increasingly many cancer patients are surviving due to early diagnoses and advances in cancer therapy. Approximately 14.5 million cancer survivors are living in the United States as of 2014, and this number is expected to grow to 19 million by 2024 [1]. Cardiovascular disease (CVD) is the second most frequent cause of death in cancer survivors, following death from cancer [2, 3]. In some cancers, a gradual increase in CVD mortality during follow-up has been demonstrated, along with stable or decreasing cancer mortality [3, 4]. Thus, the prevention of CVD is an important part of cancer survivorship [3,4,5,6]. Despite its relevance, this issue has not received adequate attention, most likely because there have been more concerns related to cancer recurrence, chemotherapy-induced cardiomyopathy, and the loss of patients during the transition period from tertiary care to primary care.
The triglyceride-glucose (TyG) index has been introduced as a surrogate marker of insulin resistance that can be easily calculated using fasting glucose and triglyceride levels without sampling serum insulin [7, 8]. Previous studies have shown that the TyG index was associated with metabolic disease, subclinical atherosclerosis, and CVD [9,10,11,12,13,14]. However, the association between the TyG index and CVD in cancer survivors, who are at higher risk for subsequent CVD [2, 15, 16], is still unknown. Understanding the association between the TyG index and CVD will help us identify individuals with high CVD risk among cancer survivors, who need adequate attention and a proactive preventive strategy for subsequent CVD.
Therefore, we conducted the present study to identify the association between the TyG index and future CVD development in a large population-based cohort of cancer patients who survived for more than 5 years beyond the diagnosis of cancer and who were initially free of CVD.
Methods
Study database and study population
We used the Korean general health screening database linked with the Korean National Health Insurance Service (NHIS) database. The NHIS provides mandatory health insurance for 97% of the Korean population and nationwide biennial health screenings. The NHIS database contains data regarding demographic characteristics, diagnosis by International Classification of Disease (ICD) codes, prescriptions, death, and health screening examination information (anthropometric measurements, blood pressure, laboratory tests, and health questionnaires) [17].
We identified 18- to 99-year-old individuals who were diagnosed with cancer during 2002–2005 and received health screening examinations during 2009–2010. The follow-up started on January 1, 2011 (the index date). An individual was defined as having cancer if he or she was hospitalized with an ICD code for cancer (C00-97) or received outpatient or inpatient care with a critical condition code for cancer (V193, V194). We excluded those with missing values for the health examinations, prescription of triglyceride-lowering agents (fibrates, niacin, or omega-3), known CVD or diabetes before the health examination, and death before the index date. The purpose of excluding individuals with known diabetes (potentially on glucose-lowering agents) or those on triglyceride-lowering agents was to examine the association in persons not receiving treatment for lowering fasting glucose or triglyceride levels. A total of 155,167 cancer survivors constituted the final study population. A detailed flowchart of the study population is provided in Additional file 1: Fig. S1. The current study was approved by the institutional review board, and the requirement for informed consent was waived, as anonymized data were provided by the NHIS under the strict confidentiality protocol.
Data collection
Venous samples, which included fasting glucose and lipid profiles, were obtained after overnight fasting [18]. Blood pressure was measured after ≥ 5 min of rest at least twice by trained staff members. Body mass index (BMI) was calculated as weight divided by the square of height (kg/m2). Data on alcohol consumption, smoking status, physical activity, and known CVD were collected via self-reported questionnaire [19]. Health examinations and data collection followed a standard protocol documented by the Ministry of Health and Welfare. More detailed information regarding the NHIS health examinations can be found elsewhere [17]. Household income status was stratified based on the quartile of all NHIS beneficiaries.
TyG index calculation and the study outcomes
The TyG index can be calculated as follows: loge [fasting triglyceride (mg) \(\times\) fasting glucose (mg)/2] (7,8). The study population was categorized into 6 groups by the level of TyG: < 8 (reference), 8–8.4, 8.5–8.9, 9.0–9.4, 9.5–9.9, and ≥ 10. A primary cardiovascular event was defined as hospitalization due to major CVD (either ischemic heart disease [IHD, I20–I25], stroke [I60–I69], or heart failure [I11, I13, I255, I42, I50]). Additionally, we explored the association between the TyG index and event subtypes (hospitalization due to acute myocardial infarction [AMI, I21], ischemic stroke [I63], and hemorrhagic stroke [I60–I62]). The validity and accuracy of the codes for CVD from the NHIS data have been tested through the formation of the Event Validation Committee, and 93% of AMI events between 2008 and 2011 were validated [20]. Another study reported that > 90% of ischemic stroke and intracerebral hemorrhage codes were validated by medical records [21]. The study population was followed until the first cardiovascular event or the end of the study (December 2020).
Statistical analysis
The baseline characteristics of study population were described as the mean ± standard deviation for continuous variables or number with percentage for categorical variables. Cox proportional hazards models were applied to evaluate the associations of the TyG index with cardiovascular events and to calculate hazard ratios (HRs), after adjustment for potential covariates, such as age, sex, household income, behavioral factors (alcohol consumption, smoking status, and physical activity), and cardiometabolic factors (systolic blood pressure, body mass index, lipid-lowering medication use, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol). Subgroup analyses were performed by age and sex. Assuming linear association, HRs per 1-unit increase in the TyG index were evaluated. Cochran’s Q statistic was used to identify the presence of interactions in the magnitude of the HR between each subgroup. Restricted cubic spline curves were created with 3 default knots at the 10th, 50th, and 90th percentiles. As an exploratory test, the associations of individual fasting glucose and triglyceride levels with primary cardiovascular events were evaluated, respectively. For this analysis, fasting glucose levels was divided into 4 categories (< 100 [reference], 100–125, 126–139, and ≥ 140 mg/dL) and fasting triglyceride levels into 5 categories (< 50 [reference], 50–99, 100–149, 150–199, 200–499, and ≥ 500 mg/dL). A 2-sided P value of < 0.05 was considered to indicate statistical significance. All statistical analyses were performed using SAS version 9.4.
Results
Baseline characteristics of the study population
Overall, 155,167 cancer survivors (mean age 59.9 ± 12.0 years, female 59.1%) comprised the study population. Their baseline demographic and biochemical profiles are presented in Table 1. In our cohort, stomach (18.8%), thyroid (15.6%), breast (13.9%), and colorectal cancer (11.7%) were the most common types of cancer. As the TyG index increased, gradual increases in systolic blood pressure, BMI, and total cholesterol were observed, as well as a gradual decrease in high-density lipoprotein cholesterol. Likewise, the high TyG index group exhibited more alcohol consumption, a higher proportion of current smokers, and less physical activity. During a median 10 years (mean 9.6 years) of follow-up, 13,279 CVD hospitalizations occurred.
Associations between the TyG index and cardiovascular events
For primary cardiovascular events, a gradual risk elevation was observed across the TyG index levels. Compared to a TyG index < 8, the HRs were 1.08 (95% CI 1.01–1.14) for a TyG index of 8.0–8.4; 1.10 (95% CI 1.03–1.17) for a TyG index of 8.5–8.9; 1.23 (95% CI 1.15–1.31) for a TyG index of 9.0-9.4; 1.34 (95% CI 1.23–1.47) for a TyG index of 9.5–9.9; and 1.55 (95% CI 1.35–1.79) for a TyG index ≥ 10 after controlling for age, sex, behavioral factors, and other laboratory findings (Table 2). Subgroup analyses by age and sex also revealed similar positive associations (Additional file 1: Table S1). As a sensitivity test, we performed the same analyses after excluding those who died in the first 3 years of follow-up and found similar results (Additional file 1: Table S2).
When we analyzed the associations of the TyG index with subtypes of CVD, graded positive associations between the TyG index and clinical outcomes were particularly evident for atherosclerotic cardiovascular disease (ASCVD), such as IHD (including AMI) and ischemic stroke, but not for hemorrhagic stroke or heart failure (Additional file 1: Table S3). For instance, the adjusted HRs for AMI were 1.23 (95% CI 0.97–1.55) for a TyG index of 8.0–8.4; 1.40 (95% CI 1.11–1.77) for a TyG index of 8.5–8.9; 1.75 (95% CI 1.36–2.23) for a TyG index of 9.0–9.4; 2.07 (95% CI 1.53–2.80) for a TyG index of 9.5–9.9; and 2.58 (95% CI 1.65–4.02) for a TyG index ≥ 10. The adjusted HRs for ischemic stroke were 1.13 (95% CI 1.02–1.25) for a TyG index of 8.0–8.4, 1.15 (95% CI 1.04–1.28) for a TyG index of 8.5–8.9, 1.34 (95% CI 1.20–1.51) for a TyG index of 9.0–9.4, 1.56 (95% CI 1.35–1.82) for a TyG index of 9.5–9.9, and 1.92 (95% CI 1.53–2.41) for a TyG index ≥ 10 compared to the reference group with a TyG index < 8. Conversely for hemorrhagic stroke and heart failure, most of the TyG categories included the null with P values > 0.05, indicating a non-significant association.
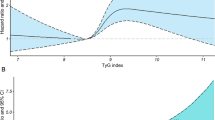
A restricted cubic spline curve for primary cardiovascular events showed a similar graded positive association to that observed in the categorical analysis (Fig. 1). In particular, the slope of association was steeper and the linear association was more apparent for ASCVD subtypes including AMI and ischemic stroke (P value for non-linearity > 0.05) than for non-ASCVD subtypes (Fig. 1 and Additional file 1: Fig. S2).
HRs for cardiovascular events using spline analyses. Restricted cubic splines of the TyG index with 3 knots (10th, 50th, and 90th percentiles) and 8.0 as a reference were used. HRs and 95% CIs were calculated using Cox proportional hazards models after adjustment for sex, age at baseline, smoking status, alcohol consumption frequency, physical activity, household income, systolic blood pressure, body mass index, lipid-lowering medication use, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol. HR: hazard ratio; TyG index: triglyceride-glucose index
When we assumed a linear association, each 1-unit increase in the TyG index was associated with a 16% increase in primary cardiovascular events (HR 1.16; 95% CI 1.12–1.19). The association was the strongest for AMI. Each 1-unit increase in the TyG index increased the risk of hospitalization for AMI by 45% (HR, 1.45; 95% CI 1.30–1.62), for ischemic stroke by 23% (HR 1.23; 95% CI 1.17–1.30), for IHD by 20% (HR 1.20; 95% CI 1.14–1.26), and for overall stroke by 13% (HR 1.13, 95% CI 1.08–1.19), after adjustment for baseline covariates. The associations between TyG index and primary cardiovascular events did not differ between subgroups (Pinteraction > 0.05), except for glycemic status, wherein a stronger association was found in higher glycemic group (Pinteraction=0.003) (Figs. 2 and 3).
HRs per 1-unit increase in the TyG index for primary cardiovascular events by confounders. HRs and 95% CIs were calculated using Cox proportional hazards models after adjustment for the same variables as in Fig. 1. HR: hazard ratio; TyG index: triglyceride-glucose index
HRs per 1-unit increase in the TyG index for acute myocardial infarction by confounders. HRs and 95% CIs were calculated using Cox proportional hazards models after adjustment for the same variables as in Fig. 1. HR: hazard ratio; TyG index: triglyceride-glucose index
Exploratory analyses: associations of individual fasting glucose and triglyceride levels with primary cardiovascular events
Regarding fasting glucose, risk elevation was only evident for fasting glucose levels ≥ 140 mg/dL after adjustment for baseline covariates compared to the reference group with fasting glucose levels < 100 mg/dL (Additional file 1: Table S4). For triglyceride levels, a potentially gradual risk elevation was demonstrated, and triglyceride levels ≥ 500 mg/dL were associated with a 59% higher risk of primary cardiovascular events (HR 1.59, 95% CI 1.26–2.01) compared to the group with triglyceride levels < 50 mg/dL (Additional file 1: Table S4). When 6 combined fasting glucose-triglyceride categories were analyzed, the group with fasting glucose ≥ 140 mg/dL and TG < 100 mg/dL was not associated with higher risk, while the group with fasting glucose ≥ 140 mg/dL and TG ≥ 500 mg/dL was associated with a 2.63-fold higher risk (Additional file 1: Table S5).
Discussion
The current study demonstrated that the TyG index was positively associated with cardiovascular events in a dose-dependent manner in a large nationwide cohort involving over 150,000 cancer survivors. Similar findings were observed across different age and sex subgroups. Notably, graded positive associations were evident for ASCVD subtypes, such as IHD, AMI, and ischemic stroke, but not for hemorrhagic stroke or heart failure. Furthermore, the slope of the association was steeper for AMI and ischemic stroke. To our knowledge, the current study is the first to demonstrate the risk of CVD occurrence based on the baseline TyG index among cancer survivors.
IHD, especially AMI, had a strong positive association with the TyG index in cancer survivors. Several studies have prospectively examined the association in non-CVD patients [22, 23] and in the general population [14, 24,25,26,27]. In studies of the general population, the TyG index was generally positively associated with IHD events, although the effect size varied across studies. A study that showed the strongest potential association (HR for the highest quartile = 2.28, compared to the lowest quartile) did not adjust for lipid levels [26]. A US study in male non-CVD patients found that the association of higher IHD mortality with a high TyG index disappeared after further adjustment of non-HDL-cholesterol [23]. In the current study, the associations were strongly maintained after adjustment for lipid levels. It is difficult to compare the effect size of the current study with those of previous studies due to differences in the outcome definition and, especially, categorizations of the TyG index. A large-scale study with 5,593,134 individuals from the Korean general population reported an HR of myocardial infarction (ICD-10: I21-I22) for the highest quartile of 1.31, compared to the lowest quartile, while, in the current study, the HRs associated with a TyG index ≥ 10 were 1.67 for IHD (ICD-10: I20-I25), and 2.58 for AMI (ICD-10: I21). Overall, the pattern of association—namely, a positive association—was similar, but whether there are differences in the effect size between general population and cancer patients requires further study to confirm.
Ischemic stroke showed a positive association with the TyG index in cancer survivors. Compared to IHD, fewer prospective studies have examined the associations in CVD patients [28] and in the general population [27, 29, 30]; in those studies, the TyG index was independently associated with a higher risk of ischemic stroke, in accordance with the current study. Among studies reporting both myocardial infarction and ischemic stroke simultaneously [27, 28], the potential associations were stronger for myocardial infarction than for ischemic stroke, in line with the current study. Regarding the strength of associations, there seemed to be some differences among studies, including ours [27,28,29,30]; the Rural Chinese Cohort Study reported an HR of 1.95 for the highest quartile of the TyG index compared to the lowest quartile [30], while the corresponding HR was 1.30 in the Kailuan study [27]. For hemorrhagic stroke, limited studies exist. Two research groups independently reported no associations for intracerebral hemorrhage in the same Chinese general population [27, 29], which was reaffirmed in our study. Hemorrhagic stroke (intracerebral hemorrhage or subarachnoid hemorrhage) is known to be affected by hemodynamic factors, rather than atherosclerotic factors. In this regard, it is considered that the TyG index, an index for insulin resistance, does not sufficiently reflect the pathophysiology of hemorrhagic stroke. Considering differences in the association between ischemic and hemorrhagic strokes, the association between TyG index and overall stroke may vary across ethnic and regional groups with different distribution of stroke subtypes.
As for heart failure, no prior studies have tested the association between the TyG index and heart failure; furthermore, inconsistent associations between insulin resistance and heart failure have been reported [31,32,33]. In the current study, no significant association was demonstrated with regard to heart failure. One plausible explanation is that heart failure is a complex clinical syndrome that originates from different etiologies.
In cancer survivors, the TyG index had clear positive-graded associations with ASCVD, such as IHD and ischemic stroke events. During recent decades, cancer outcomes have substantially improved [34, 35], and an increasing number of cancer survivors are experiencing morbidity and mortality originating from CVD [1,2,3, 36]. However, cancer patients have traditionally been excluded from most clinical trials for lipid lowering, anti-diabetes, or anti-hypertensive agents. Furthermore, metabolic parameters, such as lipid profile or glycemic status, have still not been adequately addressed and tend to be undertreated in cancer survivors. One study revealed that over half (61.7%) of lung cancer survivors for whom statin therapy was indicated were not on treatment [37]. Moreover, counseling about a healthy lifestyle (diet, exercise, or smoking) was infrequently performed among cancer survivors compared to those without cancer [38]. In this context, a well-designed large cohort study might be helpful for the understanding of this important but under-addressed health issue. Furthermore, our results imply that cancer survivors warrant proactive surveillance and management for CVD, just like the general population.
In the current study, we categorized the TyG index based on absolute values (not relative values such as quartiles) anticipating that our study data could be used as a reference in practice. Most previous studies categorized the TyG index level as tertiles or quartiles [10,11,12,13,14, 25,26,27,28,29, 39]. Although such a method can guarantee the same sample size across the groups, the cut-off values might be different across study populations, limiting their use in practice.
The precise mechanism underlying the association between an elevated TyG index (insulin resistance) and CVD events is not clear. However, several possible explanations have been suggested. First, insulin resistance is linked to endothelial dysfunction. Insulin exerts vascular actions (stimulation of the production of nitric oxide by the endothelium) beyond its metabolic actions. Thus, nitric oxide deficiency in insulin resistance could lead to insufficient vasodilation, inflammation, glucotoxicity, and lipotoxicity, resulting in a vicious cycle [40, 41]. Second, insulin resistance could stimulate hyperplasia and hypertrophy of smooth muscle cells in arterial walls, which could play a role in the development of CVD. High levels of insulin function as a potent growth factor that affects the MAPK pathway, leading to stimulation of vascular smooth muscle cell growth and activating inflammatory pathways [41, 42]. Third, insulin resistance is associated with atherogenesis (plaque progression and rupture) [10, 11, 39]. Finally, insulin resistance is associated with a cluster of metabolic abnormalities (visceral obesity, hypertension, dyslipidemia, and nonalcoholic fatty liver disease). Each component could independently contribute to the development of CVD [40].
Several limitations of the current study need to be discussed. First, the observational study design itself limits causal inferences. However, in the absence of randomized controlled trial data for metabolic profiles among cancer survivors, well-designed large population-based real-world data could play an important role. Second, we did not have data for insulin; thus, the TyG index could not be directly compared with the hyperinsulinemic-euglycemic clamp test or HOMA-IR. Although the hyperinsulinemic-euglycemic clamp test is the gold-standard method for evaluating insulin resistance, it entails additional sampling and costs, limiting its wide application at the population level. Conversely, the TyG index is a feasible and cost-effective index that uses already sampled fasting glucose and triglyceride levels. Moreover, it has reliable sensitivity and specificity for identifying insulin resistance [7, 43]. Several previous studies also have shown that the TyG index could better predict metabolic syndrome or subclinical atherosclerosis than HOMA-IR [8, 12, 39, 44]. Lastly, we did not evaluate whether the associations differed by cancer subtypes in the current study. Subsequent studies demonstrating the relationship between the TyG index and CVD in each cancer subtype would be valuable to solidify these results.
Conclusions
In summary, an elevated TyG index was associated with CVD events (particularly ASCVD) in a dose-dependent manner in a large nationwide cohort of 0.15 million cancer survivors. The TyG index could serve as a simple surrogate marker for the risk stratification of future CVD events in cancer survivors.
Availability of data and materials
The data supporting the findings of this study are available from the NHIS for the researchers, when their study proposal is reviewed and approved by the NHIS.
Abbreviations
- CVD:
-
Cardiovascular disease
- TyG:
-
Triglyceride-glucose
- NHIS:
-
National Health Insurance Service
- ICD:
-
International Classification of Disease
- BMI:
-
Body mass index
- IHD:
-
Ischemic heart disease
- AMI:
-
Acute myocardial infarction
- HR:
-
Hazard ratio
- ASCVD:
-
Atherosclerotic cardiovascular disease
References
DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64(4):252–71.
Youn JC, Chung WB, Ezekowitz JA, Hong JH, Nam H, Kyoung DS, Kim IC, Lyon AR, Kang SM, Jung HO, et al. Cardiovascular disease burden in adult patients with cancer: An 11-year nationwide population-based cohort study. Int J Cardiol. 2020;317:167–73.
Sturgeon KM, Deng L, Bluethmann SM, Zhou S, Trifiletti DM, Jiang C, Kelly SP, Zaorsky NG. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J. 2019;40(48):3889–97.
Bradshaw PT, Stevens J, Khankari N, Teitelbaum SL, Neugut AI, Gammon MD. Cardiovascular disease mortality among breast cancer survivors. Epidemiology. 2016;27(1):6–13.
Campia U, Moslehi JJ, Amiri-Kordestani L, Barac A, Beckman JA, Chism DD, Cohen P, Groarke JD, Herrmann J, Reilly CM et al. Cardio-oncology: vascular and metabolic perspectives: a scientific statement from the American Heart Association. Circulation. 2019;139(13):e579-e602.
Mehta LS, Watson KE, Barac A, Beckie TM, Bittner V, Cruz-Flores S, Dent S, Kondapalli L, Ky B, Okwuosa T et al. Cardiovascular disease and breast cancer: where these entities intersect: a scientific statement from the American Heart Association. Circulation. 2018;137(8):e30–e66.
Simental-Mendia LE, Rodriguez-Moran M, Guerrero-Romero F: The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299–304.
Vasques AC, Novaes FS, de Oliveira Mda S, Souza JR, Yamanaka A, Pareja JC, Tambascia MA, Saad MJ, Geloneze B: TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract. 2011;93(3):e98-e100.
Navarro-Gonzalez D, Sanchez-Inigo L, Pastrana-Delgado J, Fernandez-Montero A, Martinez JA: Triglyceride-glucose index (TyG index) in comparison with fasting plasma glucose improved diabetes prediction in patients with normal fasting glucose: the vascular-metabolic CUN cohort. Prev Med. 2016;86:99–105.
Park K, Ahn CW, Lee SB, Kang S, Nam JS, Lee BK, Kim JH, Park JS. Elevated TyG index predicts progression of coronary artery calcification. Diabetes Care. 2019;42(8):1569–73.
Lee SB, Ahn CW, Lee BK, Kang S, Nam JS, You JH, Kim MJ, Kim MK, Park JS. Association between triglyceride glucose index and arterial stiffness in Korean adults. Cardiovasc Diabetol. 2018;17(1):41.
Nam KW, Kwon HM, Jeong HY, Park JH, Kwon H, Jeong SM. High triglyceride-glucose index is associated with subclinical cerebral small vessel disease in a healthy population: a cross-sectional study. Cardiovasc Diabetol. 2020;19(1):53.
Li S, Guo B, Chen H, Shi Z, Li Y, Tian Q, Shi S. The role of the triglyceride (triacylglycerol) glucose index in the development of cardiovascular events: a retrospective cohort analysis. Sci Rep. 2019;9(1):7320.
Hong S, Han K, Park CY. The triglyceride glucose index is a simple and low-cost marker associated with atherosclerotic cardiovascular disease: a population-based study. BMC Med. 2020;18(1):361.
Abdel-Qadir H, Austin PC, Lee DS, Amir E, Tu JV, Thavendiranathan P, Fung K, Anderson GM. A population-based study of cardiovascular mortality following early-stage breast cancer. JAMA Cardiol. 2017;2(1):88–93.
Armenian SH, Xu L, Ky B, Sun C, Farol LT, Pal SK, Douglas PS, Bhatia S, Chao C. Cardiovascular disease among survivors of adult-onset cancer: a community-based retrospective cohort study. J Clin Oncol. 2016;34(10):1122–30.
Seong SC, Kim YY, Khang YH, Park JH, Kang HJ, Lee H, Do CH, Song JS, Bang JH, Ha S, et al. Data resource profile: the national health information database of the National Health Insurance Service in South Korea. Int J Epidemiol. 2017;46(3):799–800.
Jung MH, Yi SW, An SJ, Balkau B, Yi JJ, Kim H. Complex interaction of fasting glucose, body mass index, age and sex on all-cause mortality: a cohort study in 15 million Korean adults. Diabetologia. 2020;63(8):1616–25.
Yi SW, Park HB, Jung MH, Yi JJ, Ohrr H. High-density lipoprotein cholesterol and cardiovascular mortality: a prospective cohort study among 15.8 million adults. Eur J Prev Cardiol. 2021. https://doi.org/10.1093/eurjpc/zwab230.
Jung KJ, Jang Y, Oh DJ, Oh BH, Lee SH, Park SW, Seung KB, Kim HK, Yun YD, Choi SH et al. The ACC/AHA 2013 pooled cohort equations compared to a Korean Risk Prediction Model for atherosclerotic cardiovascular disease. Atherosclerosis. 2015;242(1):367–75.
Park TH, Choi JC: Validation of stroke and thrombolytic therapy in Korean National Health Insurance claim data. J Clin Neurol. 2016;12(1):42–8.
Sanchez-Inigo L, Navarro-Gonzalez D, Fernandez-Montero A, Pastrana-Delgado J, Martinez JA. The TyG index may predict the development of cardiovascular events. Eur J Clin Invest. 2016;46(2):189–97.
Vega GL, Barlow CE, Grundy SM, Leonard D, DeFina LF. Triglyceride-to-high-density-lipoprotein-cholesterol ratio is an index of heart disease mortality and of incidence of type 2 diabetes mellitus in men. J Investig Med. 2014;62(2):345–9.
Barzegar N, Tohidi M, Hasheminia M, Azizi F, Hadaegh F. The impact of triglyceride-glucose index on incident cardiovascular events during 16 years of follow-up: Tehran Lipid and Glucose Study. Cardiovasc Diabetol. 2020;19(1):155.
Tian X, Zuo Y, Chen S, Liu Q, Tao B, Wu S, Wang A. Triglyceride-glucose index is associated with the risk of myocardial infarction: an 11-year prospective study in the Kailuan cohort. Cardiovasc Diabetol. 2021;20(1):19.
Park B, Lee YJ, Lee HS, Jung DH. The triglyceride-glucose index predicts ischemic heart disease risk in Koreans: a prospective study using National Health Insurance Service data. Cardiovasc Diabetol. 2020;19(1):210.
Liu Q, Cui H, Ma Y, Han X, Cao Z, Wu Y. Triglyceride-glucose index associated with the risk of cardiovascular disease: the Kailuan study. Endocrine. 2022;75(2):392–9.
Zhao Q, Zhang TY, Cheng YJ, Ma Y, Xu YK, Yang JQ, Zhou YJ. Triglyceride-Glucose Index as a Surrogate Marker of Insulin Resistance for Predicting Cardiovascular Outcomes in Nondiabetic Patients with Non-ST-Segment Elevation Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention. J Atheroscler Thromb. 2021;28(11):1175–94.
Wang A, Wang G, Liu Q, Zuo Y, Chen S, Tao B, Tian X, Wang P, Meng X, Wu S et al. Triglyceride-glucose index and the risk of stroke and its subtypes in the general population: an 11-year follow-up. Cardiovasc Diabetol. 2021;20(1):46.
Zhao Y, Sun H, Zhang W, Xi Y, Shi X, Yang Y, Lu J, Zhang M, Sun L, Hu D. Elevated triglyceride-glucose index predicts risk of incident ischaemic stroke: the rural Chinese cohort study. Diabetes Metab. 2021;47(4):101246.
Vardeny O, Gupta DK, Claggett B, Burke S, Shah A, Loehr L, Rasmussen-Torvik L, Selvin E, Chang PP, Aguilar D et al. Insulin resistance and incident heart failure the ARIC study (Atherosclerosis Risk in Communities). JACC Heart Fail. 2013;1(6).531–6.
Wamil M, Coleman RL, Adler AI, McMurray JJV, Holman RR. Increased risk of incident heart failure and death is associated with insulin resistance in people with newly diagnosed type 2 diabetes: UKPDS 89. Diabetes Care. 2021;44(8):1877–84.
Kalogeropoulos A, Georgiopoulou V, Harris TB, Kritchevsky SB, Bauer DC, Smith AL, Strotmeyer E, Newman AB, Wilson PW, Psaty BM et al. Glycemic status and incident heart failure in elderly without history of diabetes mellitus: the health, aging, and body composition study. J Card Fail. 2009;15(7):593–9.
Jung KW, Won YJ, Kong HJ, Lee ES. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2016. Cancer Res Treat. 2019;51(2):417–30.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33.
van Leeuwen FE, Ng AK. Late sequelae in Hodgkin lymphoma survivors. Hematol Oncol. 2017;35 Suppl 1:60–6.
Cho IY, Han K, Shin DW, Park SH, Yoon DW, Shin S, Jeong SM, Cho JH: Cardiovascular risk and undertreatment of dyslipidemia in lung cancer survivors: a nationwide population-based study. Curr Probl Cancer. 2021;45(1):100615.
Sabatino SA, Coates RJ, Uhler RJ, Pollack LA, Alley LG, Zauderer LJ. Provider counseling about health behaviors among cancer survivors in the United States. J Clin Oncol. 2007;25(15):2100–6.
Kim MK, Ahn CW, Kang S, Nam JS, Kim KR, Park JS. Relationship between the triglyceride glucose index and coronary artery calcification in Korean adults. Cardiovasc Diabetol. 2017;16(1):108.
Di Pino A, DeFronzo RA. Insulin resistance and atherosclerosis: implications for insulin-sensitizing agents. Endocr Rev. 2019;40(6):1447–67.
Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113(15):1888–904.
Wang CC, Goalstone ML, Draznin B. Molecular mechanisms of insulin resistance that impact cardiovascular biology. Diabetes. 2004;53(11):2735–40.
Guerrero-Romero F, Simental-Mendia LE, Gonzalez-Ortiz M, Martinez-Abundis E, Ramos-Zavala MG, Hernandez-Gonzalez SO, Jacques-Camarena O, Rodriguez-Moran M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347–51.
Khan SH, Sobia F, Niazi NK, Manzoor SM, Fazal N, Ahmad F. Metabolic clustering of risk factors: evaluation of triglyceride-glucose index (TyG index) for evaluation of insulin resistance. Diabetol Metab Syndr. 2018;10:74.
Acknowledgements
The authors thank the staff at the Big Data Steering Department at the NHIS for providing the data and support.
Funding
None.
Author information
Authors and Affiliations
Contributions
MHJ and SWY conceived the study design and wrote the first draft of the manuscript. SWY acquired data. SWY and JJY performed statistical analyses. SJA., JJY, SHI, SH, KHR, HOJ, and HJY interpreted the data and contributed to the revision of the manuscript. All authors have read and approved the final submitted version of the manuscript. SWY is the study guarantor and had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The current study was approved by the institutional review board of Catholic Kwandong University (IRB No: CKU-21-01-1803), and the requirement for informed consent was waived because anonymized data (provided by the NHIS following a strong confidentiality protocol) were used.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Additional Figures and Tables.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jung, MH., Yi, SW., An, S.J. et al. Associations between the triglyceride-glucose index and cardiovascular disease in over 150,000 cancer survivors: a population-based cohort study. Cardiovasc Diabetol 21, 52 (2022). https://doi.org/10.1186/s12933-022-01490-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-022-01490-z