Abstract
As layer upon layer manufacturing approaches continue to advance the development of tissue engineering and regenerative medicine scaffolds, more products that leverage additive manufacturing methods such as 3D printing and electrospinning have been commercialized for the marketplace. This is especially true for additive manufacturing. Modifications to process parameters allow optimization of mechanical properties. This expands the applicability of currently available bioresorbable materials for tissue engineering advances. This review aims to identify these areas for potential research that would advance the field, specifically focusing on the additive manufacturing of tissue scaffolds with bioresorbable materials. To date, the terms “tissue engineering” and “additive manufacturing” have accelerated in use within research publications, and the clarity of what is required has also increased. Current reports encourage imminent successes in the field of tissue engineering with new potential for biomimicry, improved patient outcomes, and established paths for regulatory compliance. Nonetheless, there are still several challenges to overcome. As outlined in this review, a successful tissue scaffold must address and optimize six (6) critical aspects of the design and performance: biocompatibility, mechanical properties, material resorption, porosity, manufacturing, and biochemical modification. Each vital perspective of a tissue scaffold was thoroughly represented in literature. However, the totality of these aspects must be considered at the onset of a novel design poised to transition the field into an advanced future due to the interconnectivity of each criterion with each other. This is especially true when providing a new device to the clinic considering the design control focus of regulatory statutes. Bioresorbable, aliphatic polyesters hold great potential to aid this progress and mitigate a portion of the trials faced. They are proven compatible with current additive manufacturing processes and boast decades of biocompatibility established through clinical use. The development process, prioritization of processing parameters, and successful navigation through regulations have been observed with products such as Osteoplug®, Restrata®, and Biowick®. These devices exemplified the critical nature of the six aspects, and most especially the first five of them. They were specifically designed to provide environments that support bio-integration at the point of use. The native tissue provides the necessary biologics to off-the-shelf scaffold structures for successful, vascularized tissue regeneration, and ultimately, patient outcomes have been improved. This review focuses on the six critical scaffold characteristics when designing tissue scaffolds with resorbable medical-grade polymers, layer-by-layer fabrication methods, and the commercialization path for the resulting medical products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction to Tissue Engineering and Scaffold Requirements
Research into tissue engineering and regenerative medicine (TERM) [1] has provided innovative therapies [2] that hold great promise to address unmet [3] medical needs. A range of additive manufacturing (AM) modes such as 3D printing provide ample opportunity for advancement of these innovations. AM allows unprecedented control over the inner architecture of a device. This was previously not possible with molding or milling methods of manufacturing. The novel modularity breathes new life into the use of established materials to meet needs that were previously left wanting. Ratheesh et al. compiled a comprehensive review of the methods, materials, successes, and remaining challenges in 2017. Vascularization is still regarded as the ultimate sign of a successful tissue scaffold [2, 4, 5]. Once cells populate a porous structure and tubular vessels can transport to and from them throughout the device, there is an inherent confidence in continued regenerative results. Conversely, the more commonly experienced human healing mechanisms form scar tissue characterized by limited vascularization [6]. Bracaglia et al. also reviewed AM techniques but focused on the complexity of biological structures and properties. Using AM design and parameter modulation to mimic native tissues with heterogeneous scaffolds was concluded a sophisticated level of control that will be required for the future of this field [3]. Even more current reviews in tissue engineering highlight the potential for machine learning and artificial intelligence to aid in material selection and process optimization [7]. To aid in clarity with these discussions, ASTM F2312 established a standardized nomenclature and terminology for tissue engineered medical products. Tissue engineering can be generally defined as the combination of resorbable scaffolds, cells, and biomolecules toward functional tissue that can be implanted to help restore lost function or damaged tissue [1]. This review is focused primarily on the tissue scaffold.
A tissue scaffold is defined as a support matrix to allow biomolecules to regenerate in a manner to replace missing or damaged tissue [1]. Though TERM devices make up < 5% of all advance therapeutics in current clinical trials, the new device development utilized public and commercial funding to target several disorders related to muscles, cardiology, skin, and bone [8]. The success of these medical devices relates to critical design specifications that can be defined into six characteristics and properties required for tissue scaffolding. Prior to satisfying regulatory requirements, a successful tissue scaffold must be.
The commercialization path for TERM devices can differ if the scaffold will be colonized with cells prior to, during, or after implantation due to how medical devices are classified by the United States Food and Drug Administration (FDA). This differentiation [22] impacts the regulatory classification [23] and therefore determines the approval pathway [8] required for commercialization.
The term “biomaterials” refers to substances used to treat humans, or animals, after injury or disease caused a need for treatment [24]. These natural or synthetic materials are defined by application and use in the multidisciplinary field of medical practice [25]. From early Egyptian sutures to current interest in TERM devices, the goal of advancing new materials [26] and promoting better outcomes for patients is measured by biomaterial performance in each specific use case [24].
The United States Food and Drug Administration (FDA) defines a medical device based on whether it is intended for use as an implant and the mode for achieving its intended purpose. A successful medical device affects the body’s function or structure without inadvertent chemical action [27]. Additional clarifications to this definition are stated within the regulations that result in the following classification: I (e.g. band-aids), II (e.g. a scaffold), and III (e.g. drug eluting stents or a scaffold with cells on it) devices. These different descriptors allow for the many overlaps used for TERM related products, see Fig. 1.
Tissue scaffolds are therefore medical devices made with resorbable biomaterials subject to some additional and novel risks not previously required for other medical devices. The current standard of care uses biological scaffolds that are either taken from the patient themselves (autografts) or from other sources that must be decellularized prior to use (allografts and xenografts). This process offers little opportunity for optimization beyond source selection, but the market has not indicated that clinicians would adopt or prefer an artificial scaffold, even when these materials offer better performance and outcome potential. This resistance to utilize synthetic alternatives has many reasons but has allowed all areas of the field, even the regulatory agencies, more time to continue improving alternative devices and their paths to the clinic until the sentiment is shifted. For example, much work has been done to specify the regulations for the release of AM-derived regenerative-type devices into the market for use [31]. Manufacturers and researchers have also continued progress toward regenerative medicine options and production processes that satisfy these improved requirements. The literature still describes a lack of novel material options to facilitate the breakthrough. Optimization of designs and validations of processes are required by regulation though [32]. The target specifications for processes and devices are often more complicated than just requiring a minimum or maximum property. Rather, most specifications for tissue scaffolds, and their associated process parameters, must navigate narrow bands between both minimums and maximums to meet precise targets. This suggests a tortuous path filled with widespread opportunity for optimization during the development and design of tissue scaffolding with currently available materials.
A Scaffold Must be Biocompatible
One characteristic critical to all medical devices [28, 29] is cellular compatibility [33], where first it must avoid negative biological responses [34]. Avoiding adverse response and promotion of successful healing is a function of biocompatibility, cytotoxicity, time scales, and proximities. Compatibility is relative to the application and the location of the implant and determined by the interaction with the host [28]. Therefore, all other devices’ characteristics could be seen as aspects of their compatibility and effectiveness with varying levels of importance. Material selection, and even the resorption rate of that material, can impact the tissue response and scale of inflammation that occurs [11]. Controlling the device-host interaction to promote a positive outcome not only avoids negative responses but also can promote incorporation and regeneration with less follow-up therapies required. A stable endothelial layer on cardiovascular implants could eliminate thrombosis in the clinic and patients would not require antiplatelet therapy [9]. Use of natural, biological materials [17] and hydrogels [10] can present ideal environments for new cells but can also result in foreign body responses where decellularization was incomplete. Use of donor cells from the patient allows more appropriate cells to be implanted, though physiochemical properties of the scaffold are still required for success [12]. Due to the historical failure rates of biologics though [35, 36], synthetic scaffold structures may be necessary. Synthetic, bioresorbable polymers can provide a relatively lower cost option with better outcomes (lower inflammation and less infections) [14]. Understanding, predicting, manipulating, and biasing cell responses to their environment have long dominated stem cell research. Using scaffold designs to guide cell responses has gained great interest [20]. Compatibility could be maximized through optimization of properties derived from synthetic biomimicry combined with biologically sourced materials [18]. Material selection from established synthetic polymers with an established history of biocompatibility can aid ensuring success. There are many 3D printing materials that have gained popularity, such as poly lactic acid (PLA), that have an history of biocompatibility in the clinic [13]. AM with these materials has proven capable of producing devices without toxic byproducts that can be used in clinic with low rates of inflammation and rejection [15]. Acceptable benefits versus risks are an integral part of all medical design, especially with implanted devices and processing selections such as sterilization [37, 38]. Current medical device regulations serve to, in part, ensure that patient safety is prioritized from the earliest phases of design and development [8]. It is necessary for biocompatibility to be a critical design input with associated design output verifications in place. This ensures confidence that the device has and will perform as intended with as optimum a patient outcome as possible [10]. Mechanical biocompatibility has long been known as a critical aspect of biocompatibility as discussed by Shalaby in the early 1990s [39].
The Mechanical Properties Must be Optimized for the Specific Application of a Scaffold
For most implant devices, the mechanical properties hold tremendous importance in the design [40,41,42] from material selection [28, 41, 43, 44], mode of manufacturing [43,44,45,46], and design features [40, 42, 43, 45, 47]. While there are many contrived considerations associated with tissue scaffolds, the mechanical properties have always been on every list [12]. It has been noted that the mechanical strength and a perception of “feel” usually drive surgeons’ responses to a newly proposed device. A scaffold must provide the necessary support for healing [15] but also not be too stiff, resulting in other complications and risks [19]. Also, the viability of AM for manufacture of medical devices has often been concluded as being currently limited by the strength of available commercial materials [48]. AM methods have been used to mimic the arrangement and mechanical properties of native biology. This is especially powerful with the range of resorbable materials and potential modulation of each with AM parameters to target many different tissues [13]. While the material base properties known to the field may not seem optimum, AM control of geometries has been utilized to tailor metamaterial properties that mimic tissue properties [16]. A durable and supportive scaffold that is flexible enough to reproduce biological functions has aided in wound healing. Electrospinning the long established polylactide-co-glycolide (PLGA) provided the ideal tensile modulus of 40–80 MPa to mimic native skin properties [14]. The specificity of the mechanical property requirements is determined by the anatomical location [49,50,51,52], the physiological environment throughout the treatment [51,52,53,54,55], as well as the method of treatment [12, 49, 50].
While also impacting the compatibility of the device, the surface properties and mechanics can also impact cellular growth, proliferation, and even drive targeted differentiation. However, implantation methods may require the scaffold to also have sufficient suture-pull-out strength in addition to the primary performance characteristics. Contemporary literature continues to report that mechanical properties have been shown to be impacted by with processing parameters [56,57,58,59,60]. Included in the specificity of mechanical requirements is the need for the properties of a scaffold to change with the tissue as it regenerates [11].
A Scaffold Should be Bioresorbable Relative to the Rate of Tissue Regeneration
Resorbability is a synonymous term with bioresorbable in this context and refers to material performance that allows the device to be removed from the treatment area naturally, without additional procedures or risks of long-term complications and risks. Following bioresorption, only the host tissue remains. This has been established and routine for 40 years [10]. Design of a bioresorbable scaffold begins with material selection. Many materials available to AM processes, e.g. PLA, are susceptible to hydrolysis and established in bioresorbable applications [13]. This is especially true when considering lactide-based copolymers that can allow optimized degradation rates. A range of resorption rates could provide variation in the scaffold structure and its porosity relative to cell infiltration rates, optimizing proliferation [14]. Ideally, the timing of this occurs as the native tissue replaces the engineered scaffold [61, 62], first providing support [63,64,65,66,67], and then the appropriate space [14, 66, 68,69,70]. There are many types of resorption available for medical devices [28, 66], but due to the prevalence of bulk degrading aliphatic polyesters in AM methods [67], these polymers that degrade into smaller molecules by hydrolysis of their ester bonds [62] are the focus of this review. These materials are also predisposed to hydrolysis by bulk degradation, versus surface erosion, presenting a more consistent surface for cellular proliferation [11]. Resorbability is an important aspect of the compatibility and mechanical property requirements for a scaffold that can also impact proliferation and many other host interactions during and after healing [12]. The goal of choosing a bioresorbable material is to leave only the desired living tissue after healing [15].
A Scaffold Must Exhibit Optimized Porosity for the Specific Application
In balance with mechanical properties of the scaffold and the concept of biomimicry, porosity is a key characteristic [12] attributed to general functions of the scaffold such as diffusion [15] and mass transport [68]. This requires specific pore sizes and interconnectivity of the pores [63, 65, 69]. The required pore size is a function of cells’ ability to navigate and grow into and through the scaffold. A minimum pore size of 10 × the diameter of a cell could be considered based on chromatography diffusion of molecules into pores [71, 72]. For endothelial cells, this would mean a pore diameter of approximately 100 microns [11]. Biochemicals such as nutrients for the cells must also be moved to and from the cell through the scaffold [10]. Depending on the surface properties of the scaffold struts and the hydrophobicity of the scaffold material, the pores could be optimized to promote mass transfer into and out of the scaffold volume. To accomplish both requirements, a range of pore sizes would be needed in a single scaffold [73]. A range of porosity can be included in device design through computer modeling of the scaffold, already an initial step for AM [74]. While this seems contrary to many manufacturing techniques that target monodisperse scaffold structures, it aligns well with AM methods [75], and the observed pore structure of the native extracellular matrix [14] that tissue engineering strives to replicate. This biologically inspired architecture has brought interest to the varying modes of AM for TERM. However, it is still reported that the future of clinical use depends on further improvement to materials despite many successful scaffold materials reported [12, 76,77,78,79,80,81].
A Scaffold Must be Manufactured at a Scale Relative to the Specific Application
In conjunction with design, the manufacturing of a scaffold device must be positioned on a scale between commercial production and custom medicine. The potential for manufacture by multiple modes of AM has been thoroughly investigated and evaluated at a range of scales [77, 81], from industrial perspectives to large production volumes [78, 82] as well as the potential for customized medicine produced in surgery [79, 80]. The FDA has recently began preparing for regulation of AM produced medical devices [27, 83]. Producing large-scale or patient-specific customizations presents unique challenges to AM scaffold structures [8]. The primary requirement for producing medical devices remains on the documentation side of the regulations though [10]. Personalized medicine could be possible if the site of production, the hospital or surgical clinic, meets the regulatory requirements for facility that abides current good manufacturing practice (cGMP) [13]. While the ideal strategy sought in most of the recent literature has been new materials, the established history of commercially available bioresorbable polymers used in sutures and medical textiles does support the adoption of them into TERM. Similarly, the parallels between fused deposition modeling (FDM) and melt extrusion methods of suture manufacturing would also support the approval pathway for devices that utilize this AM mode of production. The preclinical phase of developing a novel medical device is regulated to contain specific steps such as documented design inputs, reviews, and outputs that occur prior to verification, validation, and regulatory approval [30]. This all occurs in parallel with growth of the production scale toward commercial viable production methods [84]. The scale that is considered commercially viable depends on the target market and end user. It has been reported that the ideal device would be readily available in a shelf-stable form [14]. Huge manufacturing efforts are needed to provide “off the shelf” devices for broad indications of use, while relatively boutique processes can adequately provide for specific, less common needs, and AM has also introduced the concept of custom medicine produced one at a time and even at the point of use [85].
A Scaffold Must Possess, Promote, or Support the Biochemistry for Specific Application
A porous, bioresorbable scaffold with optimized mechanical properties for the target tissue must be proven non-toxic and be biochemically ready to promote tissue growth and healing. Ideally that growth and healing would even be described as regeneration, avoiding the permanent scar tissue formation that is expected as a long-term consequence of mammalian tissue healing. Once a device can be manufactured at the appropriate scale under the required regulation approvals, adoption of TERM devices as standard of care then hinges on the impact to patients and clinical adoption. New modes of manufacturing, such as AM methods, offer exciting opportunities in parallel with these concerns inherent to the unknown nature of anything novel. Control over the inner architecture is an interesting, possibly necessary, method for producing scaffolds that can support cellular regeneration throughout the scaffold [13]. Due to the pressures and shear stress experienced by cardiovascular devices (stents, etc.), proven methods of ensuring a routinely successful application of endothelium in clinic have not been reported [9]. While synthetic polymer scaffolds have historically reported lower biochemical stimulation [15], biochemicals have been reported to boost performance of scaffolds [86].
Biochemically modified materials such as, Dermagraft®, based on the ubiquitous 90% glycolide / 10% lactide co-polymer (Polyglactin 910 or PGLA), performs more like a tissue scaffold with fibroblasts added, resulting in improved healing time and patient outcomes than the textile mesh alone. For this reason, cells must be combined with the mesh for it to serve as a scaffold [87, 88]. Clinical results have reported that artificial scaffolds did not decrease healing time but did reduce contraction in wound healing [6]. Growth factor (GF) delivery and interaction is basic prerequisite of the healing process [19], but a scaffold with optimal mechanics and morphology could be developed independently [14] from tailorable GF loading/release. Bioprinting with bioactive glass has been reported to support full-thickness skin regeneration in animal models [5]. Combining the ability of AM to produce a porous scaffold with novel materials (PLA blended with biomolecules) can meet the biomimicry needs of tissue engineering advancement [4, 7]. Physical and biochemical factors provide stimulation [47, 89,90,91,92,93], but also, the scaffold itself [94,95,96] can provide cues for endogenous cellular behavior [89,90,91,92,93,94,95,96,97]. The scaffold at a minimum must provide an environment that provides the potential for bio-integration, as opposed to bioactive. Biologic chemistry inherently can do this through its active sites, but current efforts in the field have suggested that construction of scaffolds with optimized porosity [21], mechanics, etc. can also sufficiently provide the required stimulation of cellular pathways associated with regeneration and healing [20, 98].
Review of the materials utilized in scaffold production allowed insight into how the six characteristics can be, and have been, achieved. Each of the primary modes of AM was examined with respect to the six critical characteristics. Current commercial devices that utilize these materials and processing modes offered additional understanding of how the characteristics are obtained and the challenges associated with scale and consistency of production. Finally, commercialized scaffold devices have successfully navigated the regulatory pathways that would again document and verify the existence of the six key characteristics as well as the proper manufacturing and documentation requirements.
The Use of Bioresorbable Polyesters in Additive Manufacturing and Tissue Engineering
The current standard of care differs mostly from the TERM devices reviewed in material and source [99]. Biocompatible, resorbable polymers are not always used. Biological tissue is harvested and used in the form of allografts and xenografts. The standard in surgery for soft tissue and even bone has been to use tissue from the patient themselves, if not a similar tissue from a donor or animal. The patient themselves is preferred because this avoids many of the risks associated with biological grafts, most notably foreign body reactions.
Material requirements from the critical aspects list include biocompatibility, appropriate mechanics, and resorbability. Additive manufacturing (AM) with materials that meet these requirements could provide novel means to generate the porous architectures needed for TERM devices and renew interest in using bioresorbable polymers to make those scaffolds. Molding and subtractive methods to process these materials have historically failed to provide sufficient modulation of mechanical properties beyond material selection. Due to the complex nature of structure–property relationships determined by processing, AM has also reinvigorated the potential to manipulate resorbable polymers to provide the ranges of mechanical properties and degradation rates needed to optimize TERM devices [100,101,102,103,104,105].
History of Bioresorbable Materials in Medicine
Synthetic, aliphatic, hydrolytically bioresorbable polyesters were originally developed more than a half-century ago and nearly that long ago to aid in tissue healing in the form of sutures. Vicryl®, (PGLA) suture was introduced in 1974 [106]. The use and implementation of bioresorbable devices have continued to grow. These materials were designed to match the application requirements before the concept of tissue engineering even had a name [99]. Initial conditions such as molecular weight, residual monomer, crystallinity, orientation, dimensional ratios, and process history (e.g. sterilization) can affect the rate of changes. Below certain cross-section dimensional boundaries, these materials all undergo bulk erosion where water attacks the polymer (as well as enzyme degradation of the materials), breaking it into smaller segments of lower molecular weight, and strength loss that always precedes mass loss [28, 39, 107,108,109].
The desire to replace current non-degradable implants and the modularity of polymeric materials has resulted in the integration of resorbable materials into research and business of nearly every industry. Poly(ethylene terephthalate), or PET, is familiar to the public [110]. Other example polyester materials in Table 1 are the core of a family of materials known as bioresorbable polyesters.
More and more device manufacturers are replacing metals and other plastics with absorbables. While these hold promise, there are major elements still missing. Ring opening polymerization (ROP) can produce polymers using strained ring molecules as monomers. ROP has resulted in sufficient molecular weight to provide the mechanical performance required for many medical devices while alleviating concerns of long-term foreign body reactions [28, 111,112,113]. Poly(D,L-lactide) (PDLA), poly(L-lactide) (PLA or PLLA), polyglycolide (PGA), polycaprolactone (PCL), and polydioxanone (PDO or PDS) were the pioneers of bioresorbable medical device materials. The addition of trimethylene carbonate (TMC) as another comonomer further expanded the possibilities for this family of aliphatic polyesters [28, 114,115,116,117,118]. Bioresorbable sutures remain the common biomedical implementation of bioresorbable materials [119]. For decades, speculation and good intentions are all that dominated the future of the field [28, 34, 120, 121]. It has also been reported that these materials are better suited for TERM than biopolymers and many non-resorbable biomaterials. [122,123,124]
Applications of Bioresorbable Polyesters in Medicine
In addition to sutures, bioresorbable materials have been considered for use nearly anywhere non-degrading plastics are currently used. This includes food packaging, composting bags, disposable dishware, and more elaborate biomedical uses [110]. Shalaby and Burg noted in their 2004 book Absorbable and Biodegradable Polymers:
“Interest in synthetic absorbable polymers has grown considerably over the past three decades, principally because of their transient nature when used as biomedical implants or drug carriers. The genesis of absorbable polymers was driven by the need to replace the highly tissue-reactive, absorbable, collagen-based sutures with synthetic polymers, which elicit milder tissue response. This led to the early development of polyglycolide as an absorbable polyester suture. In spite of the many polymeric systems investigated as candidates for absorbable implants and drug carriers, ester-based polymers maintain an almost absolute dominance among clinically used systems and others that are under investigation.” [125]
Bone fixation, drug delivery, and tissue scaffolding are currently proving additional utilization of polyester-based bioresorbables [126]. Though commanding a growing role in medical device development, a limited number of polymers composed of linear molecular chains have dominated the literature and clinical adoption. These polymers are accepted despite challenges with processing requirements, purity, and mechanical properties. Ideal medical devices for implantation exhibit high compliance, resiliency, stability, and surface hardness. Introduction of multiaxial polymer chains and novel copolymers utilizing the previously mentioned monomers creates higher degrees of freedom while retaining the bioresorbable property. Application of this freedom has been shown to address some shortcomings of earlier materials and give new enthusiasm to investigating the remaining concerns. [127,128,129,130]
The use of polymers in the medical industry is as wide as any other modern industry, but bioresorbable polymers are inherently removed from the body without additional surgeries or risks of long-term negative responses [11, 131], and bioplastics are being used and developed by standard processes [120]. The criteria for implementation of bioresorbable materials are not trivial though. A change to the material of a medical device requires re-approval from regulatory bodies that expect properties of the new material to meet or exceed that of the previous version. Even exceeding properties can raise concerns related to unknown consequences of any change. Better alignment with local biological properties is preferred but may require clinical proof of improvement that is still met with surgical skepticism related to nothing more than the change itself. Polymeric medical devices are currently prevalent due to advances in polymer processing technologies that allowed sufficient and controlled strength with lighter weight and greater flexibility [11, 99, 132, 133]. These techniques included fiber processing to create bioresorbable sutures. The rate of bioresorbable materials’ removal from the implant site, and subsequently the patient, through exhaled carbon dioxide and excreted small molecules allows another aspect of control and engineering required for each device application [11, 28]. Gajjar and King claimed the forces that are advancing degradable materials for bioengineering include the inherent long-term benefits to biocompatibility and growing technologies that require the use of bioresorbable materials. Permanent implants pose long-term risks that may require additional surgery for device removal. Novel biomedicine has a focus on TERM, controlled drug release, and gene therapy that all could benefit from, or require, the use of bioresorbable materials [126].
Features of Bioresorbable Polyesters Aiding in Tissue Engineering Success
The differences in the degradation rates of each bioresorbable polyester relate to the diffusion, crystallinity, etc. discussed, but are dominated by the local chemistry of the ester group. Each monomer provides a different steric environment for hydrolysis [134]. The access of molecular moisture to the functional group was shown to correlate to the water contact angle observed for each material. Copolymers of varied ester groups allowed optimization of degradation rate and mechanical properties [135,136,137,138,139,140]. The lactide-glycolide copolymers at varied compositions and structural organizations (PLGA or PGLA) were thought to have solved the issues related to the homopolymers and became well established as standard of care. Current research into tissue regeneration have still used PLGA successfully [141]. The expectation is still that new materials will break open the market and use of AM [42]. The mechanical properties of the mimicked biological tissue dictate the requirements of the modern material. The wide range of biological properties requires a wide range of materials [141, 142], constructs [40], and/or methods [140] that must be developed to fully realize the medical device market shift to bioresorbable polymers.
The biocompatibility [33, 142, 143], resilience [28], potential strength [126], and well-defined degradation mechanism initiated by the moisture in the body [106] are the sources of confidence in the family of bioresorbable polyesters [144, 145]. The advantages related to avoiding explants, follow-up surgeries, and long-term implant complications should not be underestimated. Most PLA and PGA-based bioresorbable, polymeric devices with high strength characteristics are also too stiff to meet the ideal modulus and mechanical compliance characteristics for materials within many areas of the body [146]. The tensile modulus of skin is reported between 0.1 and 150 MPa, [147] but the resorbable homopolymers intrinsic properties include tensile modulus of 2000 to 5000 MPa [148]. This disjunction can prevent new cellular growth from experiencing the necessary stress and strain for optimum proliferation [149]. Most PDLLA materials that satisfy the low modulus and high mechanical compliance requirements cannot form strong enough devices to support tissues under physiological conditions prior to healing. These conclusions have had an obvious effect on the rising interest related to 1,4-dioxane-2-one monomer (PDO)-based materials, but polydioxanone materials have proven costly and very difficult to process into an ideal device for other reasons [150].
The most common acceptance of bioresorbable polymers has been previously limited to use in sutures, then related textiles (e.g. PGLA used to make Vicryl® sutures and mesh) [151]. Polydioxanone (PDO) is an aliphatic polyether-ester that has been shown to degrade in the six to ten month range while providing impressive strength and flexibility when optimally processed [152,153,154,155]. This novel polymer appeared suitable to the common requirements of medical device manufactures but was not truly new. Decades earlier, Ethicon US, LLC, a Johnson and Johnson company, introduced the polydioxanone homopolymer PDS® suture, PDS® II, and a textile mesh version [151].
Polycaprolactone (PCL) is another material in this polymer family of resorbable materials. Its use is also already established in 3D printing. While the lower modulus properties can offer soft tissue mimicry potential, additives may be required to increase the strength for some applications [156]. The ability to use resorbable polyesters with multiple additive manufacturing modes has accelerated an already growing trend of transitioning clinical materials toward resorbable to avoid long-term patient risks.
Potential Bioresorbable Polyesters to Advance Tissue Scaffold Technology
PLA and PCL are well represented in AM literature, but PLA is known to be too stiff and brittle for many biological environments [149, 157,158,159]. Stiffness of PLA has generally been discussed and considered versus various tissue mechanical properties, but as resorption occurs, brittleness could also result in premature failure or sharp features to emerge, subjecting the patient to undue risks during the healing process. The brittle nature of PLA is known, but additives and copolymerization have routinely been used to overcome this mechanical property when found undesirable [159, 160]. Additionally, the majority of PLA and PCL materials commercially available for additive manufacture are not produced with the required traceability for implantable medical devices [161]. Due to the nature of the degradation process, PLA parts will increase in brittleness after implantation as well. PCL has a much lower glass transition (Tg) than PLA. It is much more mechanically compliant than PLA but sacrifices the mechanical strength of the part [156]. The strength is too low for many biological applications of tissue scaffolds. The desired and potential applications of bioresorbable polymer AM include a vast number that is not included in the currently supported portion of the field. The stronger, stiffer applications and weaker, more compliant applications leave out the stronger, higher compliance and weaker, stiff biological environments [125, 162]. The ideal material for tissue scaffold manufacture is one that mimics the mechanical strength of the target tissue after enduring the AM processing conditions. Due to the risks associated with long-term foreign body reactions, the material utilized for AM of tissue scaffolds would be best suited if it degraded into easily removed breakdown products at a rate proportional to the rate of tissue growth and biological structure formation [81, 163, 164]. The material class known as bioresorbable polymers does contain a wide array of materials with tunable mechanical properties and degradation rates [125, 165, 166]. There are very few of these bioresorbable polymers currently investigated for AM [76, 167, 168].
Controlling mechanical properties is essential to the advancement of tissue engineering but maximizing the mechanical properties possible is an inherent challenge. Tuning can only reduce the strength of a part to increase other properties without non-polymer additives [169]. The well-established properties of injection molded parts still hold competing share in the polymer processing industry as AM production requires further development to realize its full potential. Additional materials and novel combinations of materials may reveal new potential for AM and its modularity [170]. The sensitivity of bioresorbable materials generally prevents significant addition of melt processes and / or additives. Post-processing also further impacts properties [38, 171,172,173,174,175,176,177,178,179,180,181,182,183,184].
Comparing virgin PLA specimens to recycled PLA specimens tests showed that regrinding and repeating the AM process were not detrimental to the mechanical properties of the parts [185]. This information is critical to the future application of property modifiers being added to the PLA and related materials [186]. Novel materials being introduced into a new field can often confound the development of the basic sciences and understandings required for future developments based on the previous basic understanding. However, copolymers with PLA have found success in modulating material properties while allowing “significant similarity” claims for regulatory approval. Monomers such as ε-caprolactone and TMC have been copolymerized with 70% to 95% L-lactide to produce tough materials with degradation rates more aligned with tissue regeneration [28, 114,115,116,117,118]. Lactoflex® is a proprietary material offered by Poly-Med, Inc. based on 74% L-lactide, 15% TMC, and 11% PCL, see Fig. 2.
Lactoflex has provided an intermediate option for AM processes previously utilizing PLA or PCL while still satisfying the required biocompatibility and bioresorption. The potential for copolymers to fill gaps between commonly used materials without sacrificing historically provided benefits holds significant promise toward the future of successful scaffold designs.
Process Method Solutions to Challenges in Tissue Engineering
Early work in additive manufacturing (AM) of metals included mathematical modeling of the thermal events related to each layer being added. These models used known material properties of the metals to optimize for the energy inputs required to achieve ideal properties of the final part [187]. It is critical to apply these optimization techniques to AM of polymers. The energy distribution, usually in the form of heat, that dissipates away from the current deposition location induces a thermal history to all surrounding polymer material previously deposited. Molecular weight distribution (PD greater than 1) results in a range of values for each material characteristic required for accurate and precise modeling of the thermal process. Thus, empirical observations related to popular materials continue to dominate the current advancements in the field. This could be why the historical progress of the AM field has been, and will likely continue to be, dominated by the development of new or more specialized materials that enhance the technology and its potential. Continued development of novel printing materials and printing processes that expand the ability to tune mechanical properties of AM parts marks the progress that is successfully approaching the challenge of replicating parts of the human body [188].
The Many Modes of Additive Manufacturing
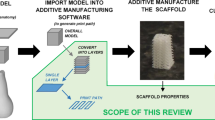
The term 3D printing refers to the layer-by-layer construction of unique shapes and products that scientifically is referred to collectively as additive manufacturing (AM). Recent reviews of 3D printing in medicine have concluded the importance of incorporating these novel modes of production with respect to biomimicry as well as how critical each phase of the process is to the success of a device. The planning and preparing prior to processing must be included with post-processing effects to properly optimize an AM process for medical device manufacturing [4]. There are several modes of manufacturing layer-by-layer though [189]. ISO/ASTM 52900 defined several process categories and identifications, see Table 2 [190].
The more common types of 3D printing are fused filament fabrication (MEX or FFF or FDM), stereolithography (VPP or SLA), and selective laser sintering (PBF or SLS) [201]. Other types of ink printing, lithography, etc. have also been used to bio-fabricate devices intended for medical applications, but these are much less represented in literature and the clinic [89]. Electrospinning (ES) has proven itself to be another layer-by-layer approach capable of producing nanoscale features very similar to the native extracellular matrix of natural tissue, see Fig. 3.
Micrographs taken with Hitachi S3400 scanning electron microscope (SEM). a and b 200 × and 2000x, respectively, Porcine small intestinal submucosa (SIS)-based decellularized tissue graft; c and d 200 × and 2000x, respectively, PLA scaffold electrospun from hexafluoroisopropanol (HFIP) solution. (Unpublished Data by authors)
The primary difference in the outputs of each AM mode is the materials available for use and the resolution of the feature size [163]. FFF is known to have a minimum layer height of 0.1 mm [197], while SLA and SLS can be lower, but ES fiber diameters are on the order of 0.001 mm and less [202, 203]. However, FFF utilizes melt extrusion of polymer materials that include materials already included in approved medical devices such as melt extruded sutures and textile meshes. This positions the FFF production mode to uniquely parallel those established regulatory pathways by removing some of the unknown aspects of bringing a novel TERM device to market [204].
Opportunities for Material Extrusion (MEX) to Advance Tissue Engineering
Medical grade, resorbable, implantable, and biocompatible material production with AM is more established than is often reported. One of the most common MEX, previously known as fused filament fabrication (FFF) materials, polylactic acid (PLA), is an aliphatic polyester very similar to the poly(lactide-co-glycolide) (PLGA or PGLA) and polydioxanone (PDS) that comprise some of the most used resorbable sutures in the clinic [34]. These sutures have been produced for half a century with melt extrusion processes that are well known and established with regulatory bodies such as CE and FDA. Therefore, melt extrusion-based AM is well situated to provide regulated device manufacturing. Though slower degrading in the body than PLGA and PDS materials, PLA is also a resorbable and compatible material that can be produced to a medical grade and has been for bone treatment options [29, 205]. Polycaprolactone (PCL) is another, even slower degrading, aliphatic polyester commonly known to be biocompatible and used as a FFF material. However, PLA has historically been reported to not have appropriate mechanical properties for many medical device needs. It can be too brittle for many soft tissue applications and too weak for many load bearing applications. PCL is inherently softer than PLA but is also much weaker than PLA [206]. While FFF has been reported as needing new materials to be a viable scaffold production method, FFF, like all layer-by-layer modes, provides control over the inner architecture of the printed device [207,208,209,210]. Mechanical, and other scaffold, properties have been reported to be an optimizable function of printing parameters [161, 187, 211,212,213,214,215,216,217,218,219,220,221,222,223]. This control allows for a level of design over the scaffold pore geometry never before possible with subtractive or molding methods of manufacturing. This characteristic of the part design is known in many AM modes as the infill parameter. Infill options include easily setting the area percentage of free space for each layer (density) [57, 161] as well as dozens of infill morphologies (pore structures) [60], each with its own potentially desirable characteristics. Larger scale FFF operations generally employ scale by duplication, driving much of the FFF hardware research toward very inexpensive printing options. Alternatively, higher priced 3D printers boast the ability to print parts faster, either by moving the extruder tip around faster or by printing with multiple extruders at once with a single printer. Either way, the entry to pilot-scale production has been found much easier with 3D printing technologies. However, full-scale levels of production manufacturing such as molding of multiple devices per second still elude the cycle time associated with AM processes. The expense of the mold equipment has been a barrier to smaller scale production though. FFF is well suited to personalized production where the hospital can send a shape or scan to a printing company, or even have 3D printers on-site at the hospital. It has even been reported that the melt extrusion process can result in sterile parts that could be immediately implantable, especially if the AM equipment is installed in the sterile field of the operating room.
3.3. Powder Bed Fusion (PBF).
PBF, previously known as selective laser sintering (SLS), utilizes the heat of a specific frequency LASER to melt or sinter small particles into a physical object. The entire print bed must be covered in powder material to facilitate the full printing range of the technique. SLS is one of the most common AM techniques for medical devices, but is generally regarded as better suited for metallic AM versus resorbable scaffold structures [83]. The SLS bed of powder presents challenges of high waste if the printing material cannot be reused. This is commonly the consensus disqualifying PBF for relatively expensive bioresorbable materials due to the materials’ sensitivity to exposure. The exposure risk of bioresorbable polymers is proportional to the surface area-to-volume ratio, and the powders utilized in PBF typically comprise very small particles with a very high surface-to-volume ratio.
Control of particle size and morphology can present additional challenges. Processing of bioresorbable materials into small and precise particle sizes has proven difficult without significant sacrifices to yield or waste. With controlled environmental parameters to limit material exposure and optimized morphology of particles, the process remains unrefined for polymeric systems [224, 225]. Metallic particles are relatively more robust and have been used for SLS for decades with other sufficient challenges reported in literature [226, 227]. It is for this reason that titanium-based SLS products overwhelmingly lead the currently approved AM medical devices, especially spinal treatments [83, 228]. These challenges await the future advancement of polymer AM systems but also provide the templates to find the solutions that are required.
The Potential for Success in Tissue Engineering with Vat Photopolymerization
Vat photopolymerization (VPP) was historically called stereolithography (SLA) and fell short early when considering AM modes for many TERM applications. Resin materials used in SLA are considered toxic, and the polymerized material output was also deemed not implantable or resorbable [229]. The catalyst content required for successful SLA printing was too high for the materials to be considered compatible with most or all tissue applications [230]. The range of mechanical properties, resins, and resin blending possible with SLA has made it a favorite in some clinics for producing, not usable / implantable medical devices, but models for planning and discussing treatment options. Of the major AM modes, SLA offers the most precise control of the part dimensions, including infill and porosity. The scale and rate of SLA print is still quite small and slow, focusing primarily on customized unique shapes with intricate detail. It is possible that the introduction of non-toxic, low catalyst resorbable resins that can be printed with standard UV curing methods and equipment would then spark the interest required for VPP equipment design to further explore the mass production of parts. The movement of the UV energy applied is not limited in the way that melt extruders for FFF are, making the potential for fast scaled VPP a seemingly feasible option in the future. These options do, however, depend on the success of new materials being developed and made available.
Electrospinning Scaffold Structures for Tissue Engineering
Electrospinning (ES) is a type of MEX where polymer is dissolved in a solvent to facilitate dispensing through a needle. ES differs from other MEX modes though because the needle is kept relatively further away from the build surface (collector). The needle is charged with a high voltage, and the collector is electrically grounded. This causes the polymer to be drawn to the collector, evaporating the solvent, and resulting in nanoscale diameter fibers to be deposited. ES constructs are generally soft, relatively weak, and difficult to scale or produce custom shapes. Resorbable materials have been used to ES biocompatible non-woven scaffold structures [231,232,233]. The properties of ES scaffolds are a function of process parameters, in part, because it is an AM mode that builds devices layer upon layer [75, 140, 234,235,236,237,238,239,240,241]. The AM method of construction provides control of the inner porosity and structure, while ES allows a unique scale for this structure. ES has proven well suited for making TERM devices that mimic the feature sizes of biological tissue and the extracellular matrix (ECM) [87, 242,243,244,245,246,247,248,249,250,251], see Fig. 3.
Use of this nanoscale structure that appears similar to natural ECM in vivo has shown improvement to healing time and outcome where other scaffold structures either did not or could not without bio-seeding. This success without biomolecules added or cell seeding has reinvigorated the field of tissue engineering with ideas for translating ES into viable production of outlook shifting TERM scaffolds that can improve the expectations for standard of care clinical options.
While ES is a mode of AM, there are also multiple modes of ES [75, 251,252,253,254]. Random, aligned, electrowriting, melted polymer, solvent dissolved polymer, and composite options have been researched and revealed that each has unique potential to contribute to the range of scaffolds that are needed to address the range of tissues biology presents. Aligned fibers mimic the structure of collagen and fibroblasts in tendons [255], while random fibers match the elasticity of skin [14]. Collagen, silk fibrils, and even live cells have been electrospun to incorporate biomolecules into the ES process and fiber structure [256]. The difference between each mode of ES is one or more of the many critical parameters that influence the ES outputs and can be summarized as hardware, process, material, and environmental parameters.
Hardware options for ES include the collector(s), needle(s), substrate(s), material(s) of construction for each component, electrical wiring, and an enclosure to allow environmental control. Environmental control is critical, and its impacts have also been well documented. The relative humidity, temperature, air pressure, exhaust flow, and the volume of space where the ES occurs have affected the fiber size, porosity, overall structure, and repeatability of the process. The process parameters noted in literature to have critical influence on the scaffold produced include the voltage (kV), dispensing / flow rate, traversing rate of the needle(s), the rate of collector(s) movement / rotation, and the distance between the needle(s) and the collector (NTDD) [236]. Post-processing can be critical to the properties and performance of ES scaffolds as well. Residual solvent impacts the mechanical properties and biocompatibility of an ES product [257]. Solvent molecules can disrupt the polymer structure and result in softening and weakening of the material. Many solvents can render the scaffold less biocompatible as well as allowing faster diffusion of water into the material, potentially speeding the degradation of aliphatic bioresorbable materials. Therefore, drying the scaffold with heat or reduced pressure after the ES process has been shown to impact mechanical properties in addition to other critical parameters of the scaffold that must be controlled to satisfy the regulations around manufacturing medical devices.
Challenges to Commercialization of Tissue Scaffold Production
Manufacturing modes such as FFF and ES have shown the ability to produce scaffold structures that mimic biological structures, providing novel opportunities for medical device design [258, 259]. The emergence of AM has provided new possibilities for design and customization of medical implants; however, 3D printed medical implants must still comply with regulations that were predominately established in the twentieth century. This design process must now introduce the use of novel processing methods to modern medical-grade materials. While the regulation path for AM and engineered tissue structures is still growing, there are success stories that have blazed a path.
Regulation of Medical Device Manufacturing
The design control requirement for tissue scaffold production is still aligned with manufacturing regulations for all medical devices. Producers are subject to surprise audit procedures to ensure compliance with the established code of federal regulations (CFR). The United States Food and Drug Administration (FDA) has stated that International Organization for Standardization (ISO) and CFR guidance documents are expected to be incorporated into quality systems to govern the manufacture of regulated devices. European conformity (CE) has ensured conformity to similar directive documents for regulation of devices made and / or sold in European countries. ISO 13485: Medical Devices has established non-negotiable methods of ensuring quality and safety. Title 21 CFR Part 820 has documented current good manufacturing practices (cGMP) as regulations for quality systems [32]. The FDA used ISO 13485 as the basis for its quality management system (QMS) regulation. The standards have outlined that manufacturers must not only define and document the outputs of the production process, but also must do so in terms that facilitate review to ensure compliance with the input requirements [32, 260]. They have established that not only must the inputs and outputs be defined and documented, objective evidence that requirements can be consistently met (Validation) must also be produced and maintained according to predetermined specifications. For materials, this level of traceability is recognized as “medical grade” due to the requirements application to raw materials in addition to the components and final devices. Several guidance documents are available through the American Society for Testing and Materials (ASTM) for what properties should be verified depending on the intended use of the device and the material composition(s) [261, 262]. ASTM has also provided testing method alignment to ensure reliability and reproducibility are quantified to acceptable levels [263,264,265,266].
Compliance with the CGMP has ensured that medical devices perform consistently, especially when mass produced. The CGMP are proven ideals for having established methods, training, and documentation throughout the production processes. Prior to production, device design must go through feasibility, then design and development phases. During feasibility, there is a specific target when selecting materials, performing prototyping, and refining the design requirements. Even this initial phase has documentation requirements for record keeping. This is so the design and development phase can refine the design, both process and product, for validation procedures to be performed using standardized instructions to produce the desired output [32, 260]. Once the design and process are ready for validation, a design freeze is implemented so that the product can be ensured consistent enough for clinical evaluations of efficacy and safety [32, 260, 267]. Further process changes after the freeze are subject to rigorous engineering change and risk assessment documentation. Re-validation may even be required for significant process changes.
This procedure could be misaligned with the production of customized devices though. AM methods have used many paths to ensure compliance with the regulations that were written in the spirit of large-scale production. To ensure traceability of outputs with customized production methods, a test sample could be made in parallel (coupon). The coupon would be a part with standardized dimensions and properties to allow verification of process performance and product quality. AM has allowed significant growth of customized and bedside medicine options; however, many surgical needs for tissue scaffolds are unvarying due to common procedures that damage and remove tissue to allow access within the patient. Standardized holes are created in the skull / bones, large blood vessels, and skin to perform laparoscopic procedures. These areas that require tissue regrowth are often the same size despite differences in patient needs. AM of standard plugs and scaffolds can provide surgeons with mass produced devices that are engineered to meet this medical need. Growth of the AM field to improve patient recovery from standard procedures can promote expansion to modular devices that could replace the surgeon’s desire or need for customized implants.
Successful Translation to the Clinic of Scaffolding Produced by Additive Manufacturing
Success with FFF to produce a device that is currently used in the clinic has been shown by 3DSystems owned Kumovis’ use of biocompatible materials at the point of care as well as with the Osteoplug® 3D printed bone plug [268, 269]. Osteopore (OPM) was founded by Dietmar Hutmacher to produce the PCL device. A pioneer in personalized medicine, Dietmar Hutmacher’s OPM Biomedical became the first company to receive FDA clearance to manufacture 3D printed patient-specific polymeric implants with its 510(k) for a cranial prothesis line for surgeons in February 2013. The company now has more clearances and is an original equipment manufacturer (OEM) for maxillofacial implants as well as its first spinal implant line. OPM also provides contract manufacturing services to third parties, impacting a broad range of 3D printed biomedical implant applications [269]. Osteoplug® is a FFF produced medical device based on PCL [270]. The mechanical properties, cell in-growth, and degradation rate are modified by compounding the monofilament with tricalcium phosphate (TCP). TCP is a common bioresorbable additive utilized to manipulate the final properties of the implant, typically to make it better suited for use with bone repair [271,272,273]. The device development was also well documented in the literature showing how the infill was used to manipulate and optimize the interconnected pore structure into multiple zones that promoted bone formation while providing sufficient mechanical properties for non-loadbearing bone [98, 274]. This also did not require the plug to be coated or treated with any biochemicals to successfully observe proliferation and differentiation of bone cells throughout the scaffold after implantation. The impact to the mechanical properties of the device due to varying the infill parameter was an interesting observation that could potentially affect the previously noted conclusions about the currently available materials for FFF. PCL has also been used for dental bone scaffolding [275]. More current FFF focused and other AM literature has reported the impact and optimization of the many processing parameters [29, 207, 218, 276,277,278,279,280]. However, conclusions are still limited to structure–property relationships and left wanting of more material options [59, 281,282,283,284].
Commercialization of ES has proven difficult in that many consider it not viable as a production process, but a small number of ES devices are in clinical use [285]. Acera Surgical Inc. has provided a randomly oriented nanofiber product. The Restrata® device has been shown to significantly improve wound healing, approaching regenerative descriptions. The device comprises PGLA and PDO materials and is available with or without fenestrations. Restrata can be applied directly to the wound site to provide a tissue scaffold to regrow native skin with more native tissue function than is expected from large and chronic wounds.
Cook Medical has successfully produced an improved version of a shoulder patch that boasts half the required healing time. PLGA is electrospun into an aligned fiber construct, reference Fig. 4, that is added to the anchor of its historically used rotator cuff implant.
The Biowick® was designed to guide healing cells and biomolecules from the implant site into the anchor site. The aligned nanoscale fibers aid in the formation of aligned fiber tissue structures in addition to the wicking action that occurs.
Niklaus Children’s Hospital is using 3D printing to both discuss surgeries with patients and allow surgeons life like models to plan and practice procedures, improving confidence and optimism for both the provider and patients. Use of the models as implants still would appear a future goal though. AM is still viewed as a prototyping technology by many.
ES continues to drive innovation that translates the gap between the lab and the clinic and the development of new tissue scaffolds and new medical devices. The paradigm and the difficulties of translating this technology into a commercial success have been reported and discussed [31].
Progress toward more viable, adoptable tissue engineering will benefit from learning more about control of the currently available aliphatic polyester bioresorbable materials. This is especially true of trends that hold across the polymer and co-polymer families within these materials. Identifying critical parameters for multiple modes of layer-by-layer manufacturing in addition to observing the impact to properties is associated with the required processing steps for manufacturing bio-resorbable tissue scaffolds, especially new and novel versions of these requirements such as modern sterilization options [286].
Outlook
The biomimicry of scaffold properties such as mechanics and porosity is expected to displace the need for seeding, coating, and adding bioactive materials prior to implantation. Fully optimized scaffolds should incorporate the native patient tissue to provide the required cells and biochemicals to the porous matrix. This will require moving beyond AM as a rapid prototyping tool and into seeing it as a uniquely capable mode of producing parts never before possible.
Emergence and use of AM with bioresorbable polymers could accelerate delivery of custom, patient-specific implants and scaffolds. There is enormous translational potential in the production of tissue engineered scaffolds using AM technologies with resorbable materials for biomedical application.
Insufficient strength, insufficient mechanical compliance, and insufficient control over mechanical properties continue to hinder further adoption of AM and bioresorbables. Layer-by-layer production controls the macrostructure and its related mechanical properties; post-processing steps manipulate and control the mechanical properties through microstructure effects; and molecular structure control via copolymerization relates to intermediate mechanical property opportunities.
The field currently requires understanding and expertise relating existing materials with novel processing methods prior to concluding that a lack of material options has limited progress in tissue engineering. Thorough investigations of additive manufacturing parameters with bioresorbable materials such as polycaprolactone and polylactides must be performed in parallel with initial design considerations. Early development phases of device design must not only establish the broad potential associated with material selections but also incorporate the impacts of post-processing and sterilization on the critical performance and even shelf-life aspects of a novel tissue scaffold. Comparative analysis of different additive manufacturing modes alongside the range of existing material options will elucidate the benefits and challenges that remain for advancement of the field.
Successful devices in clinical use have affirmed the appropriateness of bioresorbable polyester materials and additive manufacturing techniques to meet the six critical aspects of tissue scaffolds (biocompatible, mechanics, resorption, porosity, manufacturable, and biochemically stimulating). By optimizing these criteria, scaffolds have shown vascularization and full tissue regeneration in human patients at clinical scales.
Data availability
Not applicable.
References
ASTM F2312 Standard Terminology Relating to Tissue Engineered Medical Products.
G. Ratheesh, J.R. Venugopal, A. Chinappan, H. Ezhilarasu, A. Sadiq, S. Ramakrishna, J. Reddy Venugopal, A. Chinappan, H. Ezhilarasu, A. Sadiq et al., 3D fabrication of polymeric scaffolds for regenerative therapy. ACS Biomater. Sci. Eng. 3(7), 1175–1194 (2017). https://doi.org/10.1021/acsbiomaterials.6b00370
L.G. Bracaglia, B.T. Smith, E. Watson, N. Arumugasaamy, A.G. Mikos, J.P. Fisher, 3D printing for the design and fabrication of polymer-based gradient scaffolds. Acta Biomater. 56, 3–13 (2017). https://doi.org/10.1016/j.actbio.2017.03.030
C. Dong, M. Petrovic, I.J. Davies, Applications of 3D printing in medicine: a review. Ann. 3D Print. Med. (2024). https://doi.org/10.1016/j.stlm.2024.100149
Y. Liu, X. Liu, H. Guo, X. Wang, A. Li, D. Qiu, Q. Gu, 3D bioprinting bioglass to construct vascularized full-thickness skin substitutes for wound healing. Mater. Today Bio 2024, 24 (2023). https://doi.org/10.1016/j.mtbio.2023.100899
Han, S.-K. Innovations and Advances in Wound Healing, 3rd ed.; Springer, 2023.
S. Ramesh, A. Deep, A. Tamayol, A. Kamaraj, C. Mahajan, S. Madihally, Advancing 3D bioprinting through machine learning and artificial intelligence. Bioprinting 38, e00331 (2024). https://doi.org/10.1016/j.bprint.2024.e00331
K. Joyce, Z. Buljovic, G. Rosic, M. Kaszkin-Bettag, A. Pandit, Issues with tissues : trends in tissue-engineered products in clinical trials in the European Union. Tissue Eng. - Part B 29(1), 78–88 (2023). https://doi.org/10.1089/ten.teb.2022.0094
J.T. Wolfe, A. Shradhanjali, B.J. Tefft, Strategies for improving endothelial cell adhesion to blood-contacting medical devices. Tissue Eng. - Part B (2022). https://doi.org/10.1089/ten.teb.2021.0148
Atala, A.; Yoo, J. J. Essentials of 3D Biofabrication and Translation; 2015. https://doi.org/10.1016/C2013-0-18665-5.
Tissue Engineering; Fisher, J. P., Mikos, A. G., Bronzino, J. D., Eds.; CRC Press, 2007.
M.S.B. Reddy, D. Ponnamma, R. Choudhary, K.K. Sadasivuni, Polymers (Basel). 13, 1105 (2021)
S.R. Chowdhury, Y. Lokanathan, L.J. Xian, F.M. Busra, M.D. Yazid, N. Sulaiman, G. Lahiry, M.E. Hoque, Des. Manuf. (2020). https://doi.org/10.5772/intechopen.92418procedure
M.R. MacEwan, S. MacEwan, T.R. Kovacs, J. Batts, What makes the optimal wound healing material? a review of current science and introduction of a synthetic nanofabricated wound care scaffold. Cureus (2017). https://doi.org/10.7759/cureus.1736
A. Do, B. Khorsand, S.M. Geary, A.K. Salem, 3D printing of scaffolds for tissue regeneration applications. Adv. Healthc. Mater. 4, 1742–1762 (2015). https://doi.org/10.1002/adhm.201500168
K. Wang, C. Ho, C. Zhang, B. Wang, A review on the 3D printing of functional structures for medical phantoms and regenerated tissue and organ applications. Engineering 3(5), 653–662 (2017). https://doi.org/10.1016/J.ENG.2017.05.013
An, J.; Teoh, J. E. M.; Suntornnond, R.; Chua, C. K. Design and 3D printing of scaffolds and tissues. Engineering 2015. https://doi.org/10.15302/J-ENG-2015061.
J. Chakroff, D. Kayuha, M. Henderson, J. Johnson, SOJ Mater. Sci. Eng. 2(2), 1–9 (2015)
Hollister, S. J. Scaffold Design and Manufacturing. Advanced Materials. pp 3330–3342.
Lund, A. W.; Yener, B.; Stegemann, J. P.; Plopper, G. E.. Tissue Eng. - Part A 2009, 15 (3).
T. Akagi, M. Nagura, A. Hiura, H. Kojima, M. Akashi, Construction of three-dimensional dermo-epidermal skin equivalents using cell coating technology and their utilization as alternative skin for permeation studies and skin irritation tests. Tissue Eng. 23, 481–490 (2017). https://doi.org/10.1089/ten.tea.2016.0529
J.C. Valdoz, B.C. Johnson, D.J. Jacobs, N.A. Franks, E.L. Dodson, C. Sanders, C.G. Cribbs, P.M. Van Ry, The ECM: to scaffold, or not to scaffold, that is the question. Int. J. Mol. Sci. 22(23), 12690 (2021). https://doi.org/10.3390/ijms222312690
A.F. Harris, J. Lacombe, F. Zenhausern, The emerging role of decellularized plant-based scaffolds as a new biomaterial. Int. J. Mol. Sci. 22(22), 12347 (2021). https://doi.org/10.3390/ijms222212347
Institute of Biomedical Imaging, N. National Institute of Biomedical Imaging and Bioengineering Biomaterials What Are Biomaterials? 2022.
Cui, W.; Santos, H. A.; Zhang, B.; Zhang, and Y. S.. APL Bioeng. 2022, https://doi.org/10.1063/5.0078930.
D.F. Williams, On the nature of biomaterials. Biomaterials (2009). https://doi.org/10.1016/j.biomaterials.2009.07.027
Zuniga, J. M.; Major, M. J.; Peck, J. L.; Srivastava, R.; Pierce, J.; Stergiou, N. J Biomed Imag Bioeng 2017.
S.W. Shalaby, K.J.L. Burg, Absorbable and Biodegradable Polymers (CRC Press, USA, 2005)
Kurapati, S. K.; Mahendar Reddy, N.; Sujithra, R.; Kola, R.; Ramesh, G. V.; Saritha, D. Nanomaterials and Nanostructures in Additive Manufacturing: Properties, Applications, and Technological Challenges; 2023. https://doi.org/10.1002/9783527835478.ch3.
ASTM F2323–10 Standard Specification for Poly(Glycolide and Poly(Glycolide-Co-Lactide) Resins for Surgical Implants with Mole Fractions Greater Than or Equal to 70% Glycolide; 2010. https://doi.org/10.1520/F2313-10.
Morris, H. L.; Martins, J. A.; Lach., A. A.; Carr, A. J.; Mouthuy, P.-A. Translational Path for Electrospun and Electrosprayed Medical Devices from Bench to Bedside. In Biomedical Applications of Electrospinning and Electrospraying; Elsevier Ltd, 2021; pp 423–454.
21 CFR Part 820 Quality System Regulation. 2023.
E. Marin, F. Boschetto, G.J. Pezzotti, Biomed. Mater. Res.—Part A 108(8), 1617–1633 (2020). https://doi.org/10.1002/jbm.a.36930
A.M. Sousa, A.M. Amaro, A.P. Piedade, 3D printing of polymeric bioresorbable stents: a strategy to improve both cellular compatibility and mechanical properties. Polymers (Basel). 14(6), 1099 (2022). https://doi.org/10.3390/polym14061099
Integra ® Dermal Regeneration Template - Brochure. Integra LifeCciences Corporation 2012.
M. Kohlhauser, H. Luze, S.P. Nischwitz, L.P. Kamolz, Historical Evolution of Skin Grafting—a Journey through Time. Med. 57(4), 1–14 (2021). https://doi.org/10.3390/medicina57040348
Rogers, W. J. Chapter 7 Sterilisation Techniques for Polymers W.J. In Sterilisation of biomaterials and medical devices; Woodhead Publishing Series in Biomaterials, 2016; pp 1–23.
Parsons, B. J. Chapter 8 Sterilisation of Healthcare Products by Ionising Radiation: Sterilisation of Drug-Device Products and Tissue Allografts. In Sterilisation of biomaterials and medical devices; 2016; pp 1–23.
Polymers of Biological and Biomedical Significance; Shalaby, S. W., Ikada, Y., Langer, R., Williams, J., Eds.; American Chemical Society, 1994. https://doi.org/10.1021/bk-1994-0540.fw001.
D.L. Butler, S.A. Goldstein, R.E. Guldberg, E. Guo, R. Kamm, C.T. Laurencin, L.V. McIntire, V.C. Mow, R.L. Spilker, R.T. Tranquillo, Tissue Eng. - Part B 15(4), 477 (2009)
A.A. Zadpoor, J. Mech. Behav. Biomed. Mater. 70, 1–6 (2017). https://doi.org/10.1016/j.jmbbm.2017.03.018
J.S. Soares, J.E. Moore, Ann. Biomed. Eng. 44(2), 560–579 (2016). https://doi.org/10.1007/s10439-015-1477-2
T. Xu, Q. Yao, J.M. Miszuk, H.J. Sanyour, Z. Hong, H. Sun, H. Fong, Colloids Surfaces B: Biointerfaces. (2018). https://doi.org/10.1016/j.colsurfb.2018.07.004
F. Yang, V. Tadepalli, B.J. Wiley, ACS Biomater. Sci. Eng. 3(5), 863–869 (2017). https://doi.org/10.1021/acsbiomaterials.7b00094
A. Unkovskiy, S. Spintzyk, D. Axmann, E.M. Engel, H. Weber, F. Huettig, J. Prosthodont. 28, 1–9 (2017). https://doi.org/10.1111/jopr.12681
A.A. Zadpoor, Bone tissue regeneration: the role of scaffold geometry. Biomater. Sci. 3(2), 231–245 (2015). https://doi.org/10.1039/c4bm00291a
S. Moeinzadeh, S.R. Pajoum Shariati, E. Jabbari, Comparative effect of physicomechanical and biomolecular cues on zone-specific chondrogenic differentiation of mesenchymal stem cells. Biomaterials (2016). https://doi.org/10.1016/j.biomaterials.2016.03.034
M. Kathryn, G. Moroni, T. Vaneker, G. Fadel, R.I. Campbell, I. Gibson, A. Bernard, J. Schulz, P. Graf, B. Ahuja et al., CIRP annals—manufacturing technology design for additive manufacturing : trends, opportunities, considerations, and constraints. CIRP Ann. - Manuf. Technol. 65(2), 737–760 (2016). https://doi.org/10.1016/j.cirp.2016.05.004
A.D. Olubamiji, Z. Izadifar, J.L. Si, D.M.L.L. Cooper, F. Eames, D.X.X.B. Chen, B.F. Eames, D.X.X.B. Chen, Biofabrication 8(2), 1–18 (2016). https://doi.org/10.1088/1758-5090/8/2/025020
F. Pati, D.H. Ha, J. Jang, H.H. Han, J.W. Rhie, D.W. Cho, Biomaterials (2015). https://doi.org/10.1016/j.biomaterials.2015.05.043
A.J. Engler, S. Sen, H.L. Sweeney, D.E. Discher, Cell 126(4), 677–689 (2006). https://doi.org/10.1016/j.cell.2006.06.044
D.E. Discher, P. Janmey, Y.L. Wang, Science 310(5751), 1139–1143 (2005). https://doi.org/10.1126/science.1116995
K. Feng, A. Pinkas-sarafova, V. Ricotta, M. Cuiffo, Soft Matter 14, 9838–9846 (2018). https://doi.org/10.1039/c8sm01797b
S. Bose, S.F. Robertson, A. Bandyopadhyay, Acta Biomater. (2018). https://doi.org/10.1016/j.actbio.2017.11.003
C.R. Deeken, B.J. Eliason, M.D. Pichert, S.A. Grant, M.M. Frisella, B.D. Matthews, Ann. Surg. 255(3), 595–604 (2012). https://doi.org/10.1097/SLA.0b013e3182445341
S. Garzon-hernandez, D. Garcia-gonzalez, A. Jérusalem, A. Arias, Mater. Des. 188, 108414 (2020). https://doi.org/10.1016/j.matdes.2019.108414
C.J. Culbreath, B. Gaerke, M.S. Taylor, S.D. McCullen, O.T. Mefford, Materialia (2020). https://doi.org/10.1016/j.mtla.2020.100732
L. Pisanu, L.C. Santiago, J. Dantas, V. Barbosa, V. Estev, M. Luis, F. Nascimento, Polymers (Basel). 13, 1039 (2021)
G.D. Goh, Y.L. Yap, H.K.J. Tan, S.L. Sing, G.L. Goh, W.Y. Yeong, Crit. Rev. Solid State Mater. Sci. 45(2), 113–133 (2020). https://doi.org/10.1080/10408436.2018.1549977
Dave, H. K.; Patadiya, N. H.; Prajapati, A. R.; Rajpurohit, S. R. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2019, https://doi.org/10.1177/0954406219856383.
Hutmacher, D. W.; Tandon, B.; Dalton, P. D. Scaffold Design and Fabrication; Elsevier Inc., 2022. https://doi.org/10.1016/B978-0-12-824459-3.00011-1.
H. Samami, J.A. Pan, J. Mech. Behav. Biomed. Mater. 59, 430 (2016)
N. Chantarapanich, P. Puttawibul, S. Sucharitpwatskul, P. Jeamwatthanachai, S. Inglam, K. Sitthiseripratip, Comput. Math. Methods Med. (2012). https://doi.org/10.1155/2012/407805
I. Zein, D.W. Hutmacher, K. Cheng Tan, S.H. Teoh, Biomaterials 23, 1169–1185 (2002)
Savio, G.; Rosso, S.; Meneghello, R.; Concheri, G. Review article geometric modeling of cellular materials for additive manufacturing in biomedical field : a review. 2018, 2018.
D.F. Williams, The biomaterials conundrum in tissue engineering. Tissue Eng. - Part A 20(7–8), 1129–1131 (2014). https://doi.org/10.1089/ten.tea.2013.0769
Z. Wang, Y. Wang, Y. Ito, P. Zhang, X. Chen, Sci. Rep. 6, 1–13 (2016). https://doi.org/10.1038/srep20770
G.A. Wirth, D.S. Mowlds, P. Guidotti, A.A. Salibian, A. Nguyen, K.Z. Paydar, Eur. J. Plast. Surg. (2015). https://doi.org/10.1007/s00238-015-1090-5
M. Alksne, E. Simoliunas, M. Kalvaityte, E. Skliutas, I. Rinkunaite, I. Gendviliene, D. Baltriukiene, V. Rutkunas, V. Bukelskiene, Biomed Mater Res A. (2018). https://doi.org/10.1002/jbm.a.36547
C. Li, C. Guo, V. Fitzpatrick, A. Ibrahim, M.J. Zwierstra, P. Hanna, A. Lechtig, A. Nazarian, S.J. Lin, D.L. Kaplan, Nat. Rev. Mater. 5(1), 61–81 (2020). https://doi.org/10.1038/s41578-019-0150-z
G. Vydac, Hesperia, CA, USA Grace Vydac 0924, 36 (2002)
Miller, J. M. Chromatography concepts and contrasts SECOND EDITION, Second.; John Wiley & Sons, Inc., 2005.
O. Cansizoglu, D. Cormier, O. Harrysson, H. West, T. Mahale, An evaluation of non-stochastic lattice structures fabricated via electron beam melting (North Carolina State University, USA, 2006), pp.209–219
J. An, J.E.M. Teoh, R. Suntornnond, C.K. Chua, Engineering 1(2), 261–268 (2015)
W.E. King, G.L. Bowlin, Polymers (Basel). 13(7), 1097 (2021)
A. Sharma, W.A. Faubion, A.B. Dietz, Mayo Clinic. Proceed. (2019). https://doi.org/10.1016/j.mayocp.2019.03.013
M. Fera, Int. J. Adv. Manuf. Technol. (2018). https://doi.org/10.1007/s00170-017-1221-1
D.R. Eyers, A.T. Potter, J. Gosling, M.M. Naim, D.R. Eyers, A.T. Potter, J. Gosling, Int. J. Oper. Prod. Manag. 38(12), 2312–2343 (2018). https://doi.org/10.1108/IJOPM-04-2016-0200
P. Tack, J. Victor, P. Gemmel, L. Annemans, Biomed. Eng. Online (2016). https://doi.org/10.1186/s12938-016-0236-4
N. Martelli, C. Serrano, H. Van Den Brink, J. Pineau, P. Prognon, I. Borget, S. El Batti, Surg. 159(6), 1485–1500 (2016). https://doi.org/10.1016/j.surg.2015.12.017
C.N. Ryan, K.P. Fuller, A. Larrañaga, M. Biggs, Y. Bayon, J.R. Sarasua, A. Pandit, D.I. Zeugolis, Larrañ aga, A. Expert Rev. Med. Devices 12(5), 601–612 (2015)
D.R. Eyers, A.T. Potter, Computers in Industry Industrial Additive Manufacturing : A Manufacturing Systems Perspective. 93, 208–218 (2017). https://doi.org/10.1016/j.compind.2017.08.002
M. Fogarasi, K.L. Snodderly, M.A. Di Prima, Nat. Rev. Bioeng. (2023). https://doi.org/10.1038/s44222-023-00109-6
J.C. Chen, V.S. Gabriel, Proc. IEEE Int. Conf. Ind. Technol. (2016). https://doi.org/10.1109/ICIT.2016.7474872
M.H. Lee, J.A. Arcidiacono, A.M. Bilek, J.J. Wille, C.A. Hamill, K.M. Wonnacott, M.A. Wells, S.S. Oh, Tissue Eng—Part B: Rev. (2010). https://doi.org/10.1089/ten.teb.2009.0449
B. Aldemir Dikici, G.C. Reilly, F. Claeyssens, ACS Appl. Mater. Interfaces 12(11), 12510–12524 (2020). https://doi.org/10.1021/acsami.9b23100
Tuzlakoglu, K.; Reis, R. L. Tissue Eng. - Part B 2009, 15 (1).
L. Uebersax, H.P. Merkle, L. Meinel, Tissue Eng. - Part B 15(3), 263 (2009)
Y. Guan, B. Yang, W. Xu, D. Li, S. Wang, Z. Ren, J. Zhang, T. Zhang, X. Liu, J. Li et al., Cell-derived extracellular matrix materials. Tissue Eng. - Part B 28(5), 1007–1021 (2022). https://doi.org/10.1089/ten.teb.2021.0147
Z. Zhao, Y. Sun, Q. Qiao, L. Zhang, X. Xie, M.D. Weir, A. Schneider, H.H.K. Xu, N. Zhang, K. Zhang et al., Human periodontal ligament stem cell and umbilical vein endothelial cell co-culture to prevascularize scaffolds for angiogenic and osteogenic tissue engineering. Int. J. Mol. Sci. 22(22), 12363 (2021). https://doi.org/10.3390/ijms222212363
P. Wang, Y. Sun, X. Shi, H. Shen, H. Ning, H. Liu, Bioact. Mater. 6(5), 1283–1307 (2021). https://doi.org/10.1016/j.bioactmat.2020.10.014
S. Derakhshanfar, R. Mbeleck, K. Xu, X. Zhang, W. Zhong, M. Xing, Bioact. Mater. 3(2), 144–156 (2018). https://doi.org/10.1016/j.bioactmat.2017.11.008
B.C. Shah, M.M. Tiwari, M.R. Goede, M.J. Eichler, R.R. Hollins, C.L. McBride, J.S. Thompson, D. Oleynikov, Hernia (2011). https://doi.org/10.1007/s10029-010-0768-7
A.J. Kanter, De; Jongsma, K. R., Verhaar, M. C., Bredenoord, A. L., The ethical implications of tissue engineering for regenerative purposes : a systematic review. Tissue Eng. - Part B 29(2), 167–187 (2023). https://doi.org/10.1089/ten.teb.2022.0033
A.E. Jakus, A.L. Rutz, S.W. Jordan, A. Kannan, S.M. Mitchell, C. Yun, K.D. Koube, S.C. Yoo, H.E. Whiteley, C.P. Richter et al., Hyperelastic “Bone”: a highly versatile, growth factor-free, osteoregenerative, scalable, and surgically friendly biomaterial. Sci. Transl. Med. 8(358), 1–16 (2016). https://doi.org/10.1126/scitranslmed.aaf7704
D. Hu, Z. Dong, B. Li, F. Lu, Y. Li, Mechanical force directs proliferation and differentiation of stem cells. Tissue Eng. - Part B 29(2), 141–150 (2023). https://doi.org/10.1089/ten.teb.2022.0052
S. Moeinzadeh, S.R. Pajoum Shariati, E. Jabbari, Biomaterials 92, 57–70 (2016). https://doi.org/10.1016/j.biomaterials.2016.03.034
D.W. Hutmacher, T. Schantz, I. Zein, K.W. Ng, S.H. Teoh, K.C. Tan, Mechanical properties and cell cultural response of polycaprolactone scaffolds designed and fabricated via fused deposition modeling. J. Biomed. Mater. Res. 55, 203 (2001)
Biomedical Polymers; Jenkins, M., Ed.; Woodhead Publishing Limited, 2007.
T.D. Ngo, A. Kashani, G. Imbalzano, K.T.Q. Nguyen, D. Hui, Applications and Challenges. Compos. Part B 143, 172–196 (2018). https://doi.org/10.1016/j.compositesb.2018.02.012
S. Bose, D. Ke, H. Sahasrabudhe, A. Bandyopadhyay, Additive manufacturing of biomaterials. Prog. Mater. Sci. (2018). https://doi.org/10.1016/j.pmatsci.2017.08.003
Perale, G.; Hilborn, J. Bioresorbable Polymers for Biomedical Applications; 2017. https://doi.org/10.1016/B978-0-08-100262-9.18001-2.
S. Singh, S. Ramakrishna, R. Singh, J. Manuf. Process. (2017). https://doi.org/10.1016/j.jmapro.2016.11.006
I. Chiulan, A. Frone, C. Brandabur, D. Panaitescu, Recent advances in 3D printing of aliphatic polyesters. Bioengineering 5(1), 2 (2017). https://doi.org/10.3390/bioengineering5010002
J. Günther, F. Brenne, M. Droste, M. Wendler, O. Volkova, H. Biermann, T. Niendorf, Sci. Rep. (2018). https://doi.org/10.1038/s41598-018-19376-0
L.N. Woodard, M.A. Grunlan, Hydrolytic degradation and erosion of polyester biomaterials. ACS Macro Lett. 7(8), 976–982 (2018). https://doi.org/10.1021/acsmacrolett.8b00424
Treiser, M.; Abramson, S.; Langer, R.; Kohn, J. Degradable and Resorbable Biomaterials, Third Edit.; Elsevier, 2013. https://doi.org/10.1016/B978-0-08-087780-8.00021-8.
Treiser, M.; Abramson, S.; Langer, R.; Kohn, J. Degradable and Resorbable Biomaterials. In Biomaterials Science: An Introduction to Materials: Third Edition; Elsevier, 2013; pp 179–195. https://doi.org/10.1016/B978-0-08-087780-8.00021-8.
K. Ceonzo, A. Gaynor, L. Shaffer, K. Kojima, C.A. Vacanti, G.L. Stahl, Tissue Eng. 12(2), 301–308 (2006). https://doi.org/10.1161/CIRCULATIONAHA.110.956839
A. Emblem, Packaging Technology: Fundamentals (Materials and Processes; Woodhead Pub, Cambridge, 2012)
G. Odian, Principles of Polymerization (Fourth.; John Wiley & Sons Inc, USA, 2004)
J. Pretula, S. Slomkowski, S. Penczek, Adv. Drug Deliv. Rev. (2016). https://doi.org/10.1016/j.addr.2016.05.002
O. Nuyken, S.D. Pask, Polymers (Basel). (2013). https://doi.org/10.3390/polym5020361
W. Guerin, M. Helou, J.-F. Carpentier, M. Slawinski, J.-M. Brusson, S.M. Guillaume, Macromolecular Engineering via Ring-Opening Polymerization: L-Lactide/Trimethylene Carbonate Block Copolymers as Thermoplastic Elastomers. Polym. Chem. 4(4), 1095–1106 (2013). https://doi.org/10.1039/C2PY20859H
Shalaby, S. W. United States Patent - High Strength Fibers of L-Lactide Copolymers e-Caprolactone and Trimethylene Carbonate and Absorbable Medical Constructs Thereof. US007192437B2, 2007. https://doi.org/10.1021/n10602701.
Shalaby, S. W. United States Patent US6342065B1 High Strength Fibers of L-Lactide Copolymers ε-Caprolactone and Trimethylene Carbonate and Absorbable Medical Constructs Thereof, 2002.
S. Mohajeri, B.G. Amsden, ACS Appl. Bio Mater. (2021). https://doi.org/10.1021/acsabm.1c00160
H. Ajiro, Y. Haramiishi, N. Chanthaset, M. Akashi, Polym. J. 48(7), 751–760 (2016). https://doi.org/10.1038/pj.2016.35
M. Rios-Galacho, D. Martinez-Moreno, E. Lopez-Ruiz, P. Galvez-Martin, J.A. Marchal, An overview on the manufacturing of functional and mature cellular skin substitutes. Tissue Eng. - Part B 28(5), 1035–1052 (2022). https://doi.org/10.1089/ten.teb.2021.0131
Bastioli, C.; Vasile, C.; Domb, A. J.; Kost, J.; Sheva, B.; Wiseman, D. M.; Bastioli, C.; Vasile, C. Handbook of Biodegradable Polymers; Domb, A. J., Kost, J., Wiseman, D. M., Eds.; CRC Press, 1997; Vol. Volume 51. https://doi.org/10.1097/00000433-198206000-00020.
H. Tian, Z. Tang, X. Zhuang, X. Chen, X. Jing, Prog. Polym. Sci. 37(2), 237–280 (2012). https://doi.org/10.1016/j.progpolymsci.2011.06.004
E. Vonbrunn, M. Mueller, M. Pichlsberger, M. Sundl, A. Helmer, S.A. Wallner, B. Rinner, A.-C. Tuca, L.-P. Kamolz, D. Brislinger et al., Front. Bioeng. Biotechnol. (2020). https://doi.org/10.3389/fbioe.2020.604123
M.C. Wurm, T. Möst, B. Bergauer, D. Rietzel, F.W. Neukam, S.C. Cifuentes, C. von Wilmowsky, In-vitro evaluation of polylactic acid (PLA) manufactured by fused deposition modeling. J. Biol. Eng. 11(1), 1–10 (2017). https://doi.org/10.1186/s13036-017-0073-4
K.M. Patel, P. Bhanot, Plast. Reconstr. Surg. (2012). https://doi.org/10.1097/PRS.0b013e318262e186
WShalaby, S.; JLBurg, K. Absorbable and Biodegradable Polymers; 2005.
Gajjar, C. R.; King, M. W. Resorbable Fiber-Forming Polymers for Biotextile Applications; 2014. https://doi.org/10.1007/978-3-319-08305-6.
Shalaby. US6794485 Amorphous Polymeric Polyaxial Initiators and Compliant Crystalline Copolymers Therefrom, 2002.
Shalaby. US6462169 Amorphous Polymeric Polyaxial Initiators and Compliant Crystalline Copolymers Therefrom, 2002. https://doi.org/10.1016/j.(73).
Shalaby. US7070858 Amorphous Polymeric Polyaxial Initiators and Compliant Crystalline Copolymers Therefrom, 2003.
Shalaby. US7129319 Amorphous Polymeric Polyaxial Initiators and Compliant Crystalline Copolymers Therefrom. 2006. https://doi.org/10.1074/JBC.274.42.30033.(51).
Suzuki, S.; Ikada, Y. Biomaterials for Surgical Operation; Humana Press, 2012.
Pomager, J. 5 Big Trends Affecting Polymer Material AndProcess Selection For Medical Devices.
B.D. Ratner, S.J. Bryant, Annu. Rev. Biomed. Eng. 6, 41–75 (2004). https://doi.org/10.1146/annurev.bioeng.6.040803.140027
A. Gleadall, J. Pan, M.A. Kruft, M. Kellomäki, Acta Biomater. (2014). https://doi.org/10.1016/j.actbio.2013.12.039
L. Feng, B. Zhang, X. Bian, G. Li, Z. Chen, X. Chen, Macromolecules 50(16), 6064–6073 (2017). https://doi.org/10.1021/acs.macromol.7b00818
H. Tsuji, Adv. Drug Deliv. Rev. (2016). https://doi.org/10.1016/j.addr.2016.04.017
E. Castro-Aguirre, F. Iñiguez-Franco, H. Samsudin, X. Fang, R. Auras, Adv. Drug Deliv. Rev. (2016). https://doi.org/10.1016/j.addr.2016.03.010
D. Mondal, M. Griffith, S.S. Venkatraman, Int. J. Polym. Mater. Polym. Biomater. (2016). https://doi.org/10.1080/00914037.2015.1103241
P.S.P. Poh, M.P. Chhaya, F.M. Wunner, E.M. De-Juan-Pardo, A.F. Schilling, J.T. Schantz, M. van Griensven, D.W. Hutmacher, Adv. Drug Deliv. Rev. (2016). https://doi.org/10.1016/j.addr.2016.07.006
C. Thammawong, S. Buchatip, A. Petchsuk, P. Tangboriboonrat, N. Chanunpanich, M. Opaprakasit, P. Sreearunothai, P. Opaprakasit, Polym. Eng. Sci. (2014). https://doi.org/10.1002/pen.23576
M. Shokouhimehr, A.S. Theus, A. Kamalakar, L. Ning, C. Cao, M.L. Tomov, J.M. Kaiser, S. Goudy, N.J. Willett, H.W. Jang et al., Polymers (Basel). 13, 1099 (2021)
D. Pappalardo, T. Mathisen, A. Finne-Wistrand, Biomacromol 20(4), 1465–1477 (2019). https://doi.org/10.1021/acs.biomac.9b00159
Y. Ramot, M. Haim-Zada, A.J. Domb, A. Nyska, Adv. Drug Deliv. Rev. (2016). https://doi.org/10.1016/j.addr.2016.03.012
M. Kaduri, M. Poley, O. Adir, N. Krinsky, Chem. Eng. J. 340(January), 9–14 (2018). https://doi.org/10.1016/j.cej.2018.01.010
A.-C.C. Albertsson, U. Edlund, K. Stridsberg, Macromol. Symp. 157, 39–46 (2000)
D. Hutmacher, M.B. Hürzeler, H. Schliephake, A review of material properties of biodegradable and bioresorbable polymers and devices for GTR and GBR applications. Int. J. Oral Maxillofac. Implants 11(5), 667–678 (1997)
A. Kalra, A. Lowe, A. Am, Mechanical behaviour of skin : a review. J. Mater. Sci. Eng. (2016). https://doi.org/10.4172/2169-0022.1000254
Carrasco, F.; Pag??s, P.; G??mez-P??rez, J.; Santana, O. O.; Maspoch, M. L. Processing of Poly(Lactic Acid): Characterization of Chemical Structure, Thermal Stability and Mechanical Properties. Polym. Degrad. Stab. 2010. https://doi.org/10.1016/j.polymdegradstab.2009.11.045.
J.S. Bergström, D. Hayman, Ann. Biomed. Eng. (2016). https://doi.org/10.1007/s10439-015-1455-8
Gajjar, C. R.; King, M. W. SPRINGER BRIEFS IN MATERIALS Resorbable Fiber-Forming Polymers for Biotextile Applications.
Bronzino, J. D. Biomedical Engineering Fundamentals; 2006. https://doi.org/10.1017/CBO9781107415324.004.
Y. Bai, C. Ma, P. Wang, Z. Fan, W. Bai, C. Xiong, C. Tang, Polym. Compos. 33, 1700 (2012)
Bezwada, R. S.; Scopelianos, A. G. Elastomeric Medical Device. US005468253A, 1995.
Rao S. Bezwada, Park, M.; Burkard, H. G.-. United States Patent [191. 1989.
C. Jin, B. Liang, J. Li, F. Li, J. Polym. Environ. (2013). https://doi.org/10.1007/s10924-013-0613-z
M.A. Woodruff, D.W. Hutmacher, The return of a forgotten polymer - polycaprolactone in the 21st century. Prog. Polym. Sci. (2010). https://doi.org/10.1016/j.progpolymsci.2010.04.002
Y. Liao, C. Liu, B. Coppola, G. Barra, L. Maio, Di; Incarnato, L., Lafdi, K., PLA properties. Polymers (Basel). 11(1487), 1–14 (2019)
S. Farah, D.G. Anderson, R. Langer, Adv. Drug Deliv. Rev. (2016). https://doi.org/10.1016/j.addr.2016.06.012
S. Vacaras, M. Baciut, O. Lucaciu, C. Dinu, G. Baciut, L. Crisan, M. Hedesiu, B. Crisan, F. Onisor, G. Armencea et al., Drug Metab. Rev. 51(4), 570–588 (2019). https://doi.org/10.1080/03602532.2019.1642911
Farah, S.; Anderson, D. G.; Langer, R. Physical and Mechanical Properties of PLA , and Their Functions In. 2016, 0–26.
M. Mohseni, D.W. Hutmacher, N.J. Castro, Polymers (2018). https://doi.org/10.3390/polym10010040
T. Barrows, Clin. Mater. (1986). https://doi.org/10.1016/S0267-6605(86)80015-4
S.C. Ligon, R. Liska, J. Stampfl, M. Gurr, R. Mülhaupt, Chem. Rev. (2017). https://doi.org/10.1021/acs.chemrev.7b00074
J.U. Pucci, B.R. Christophe, J.A. Sisti, E.S. Connolly, Biotechnol. Adv. (2017). https://doi.org/10.1016/j.biotechadv.2017.05.007
H.Y. Ang, H. Bulluck, P. Wong, S.S. Venkatraman, Y. Huang, N. Foin, Int. J. Cardiol. 228, 931–939 (2017). https://doi.org/10.1016/j.ijcard.2016.11.258
J. Idaszek, A. Bruinink, ͆więszkowski, W. Polym. Degrad. Stab. (2016). https://doi.org/10.1016/j.polymdegradstab.2015.12.020
C.M. González-Henríquez, M.A. Sarabia-Vallejos, J. Rodriguez-Hernandez, Prog. Polym. Sci. 94, 57–116 (2019). https://doi.org/10.1016/j.progpolymsci.2019.03.001
M.P. Nikolova, M.S. Chavali, Bioact. Mater. 2019(4), 271–292 (2019). https://doi.org/10.1016/j.bioactmat.2019.10.005
P. Saini, M. Arora, M.N.V.R. Kumar, Adv. Drug Deliv. Rev. (2016). https://doi.org/10.1016/j.addr.2016.06.014
L. Liao, J. Dong, L. Shi, Z. Fan, S. Li, Z. Lu, Polym. Degrad. Stab. 111, 203–210 (2015). https://doi.org/10.1016/j.polymdegradstab.2014.11.013
G. Török, P. Gombocz, E. Bognár, P. Nagy, E. Dinya, B. Kispélyi, P. Hermann, BMC Oral Health 20(1), 1–12 (2020). https://doi.org/10.1186/s12903-020-1005-0
G.C.C. Mendes, T.R.S. Brandão, C.L.M. Silva, Ethylene oxide sterilization of medical devices: a review. Am. J. Infect. Control 35(9), 574–581 (2007). https://doi.org/10.1016/j.ajic.2006.10.014
Mendes, G. C.; Brandão, T. R. S.; Silva, C. L. M. Chapter 4 Ethylene Oxide (EO) Sterilization of Healthcare Products G.C. In Sterilisation of biomaterials and medical devices; Woodhead Publishing Series in Biomaterials, 2016; pp 1–23.
M. Savaris, V. Santos, dos; Brandalise, R. N., Influence of different sterilization processes on the properties of commercial poly(lactic acid). Mater. Sci. Eng. C 69, 661–667 (2016). https://doi.org/10.1016/j.msec.2016.07.031
Z. Dai, J. Ronholm, Y. Tian, B. Sethi, X. Cao, Sterilization techniques for biodegradable scaffolds in tissue engineering applications. J. Tissue Eng. (2016). https://doi.org/10.1177/2041731416648810
N.A. Weir, F.J. Buchanan, J.F. Orr, D.F. Farrar, A. Boyd, Biomaterials 25(18), 3939–3949 (2004). https://doi.org/10.1016/j.biomaterials.2003.10.076
Irradiation of Polymers: Fundamentals and Technological Applications, ACS Sympos.; Clough, R. L., Shalaby, S. W., Eds.; American Chemical Society, 1996.
M. Haim Zada, A. Kumar, O. Elmalak, G. Mechrez, A.J. Domb, ACS Omega 4(25), 21319–21326 (2019). https://doi.org/10.1021/acsomega.9b02889
E. Avila, de; Eo, J., Kim, J., Kim, N. P., MATEC Web Conf. 32, 883–888 (2019)
R.E. Abhari, P.A. Mouthuy, N. Zargar, C. Brown, A. Carr, J. Mech. Behav. Biomed. Mater. 2017(67), 127–134 (2016). https://doi.org/10.1016/j.jmbbm.2016.11.023
J.S. Chohan, R. Singh, Rapid Prototyp. J. 23(3), 495–513 (2017). https://doi.org/10.1108/RPJ-05-2015-0059
S. Lerouge, A. Simmons, Non-Traditional Sterilization Techniques for Biomaterials and Medical Devices (Woodhead Publishing Series in Biomaterials, In Sterilisation of biomaterials and medical devices, 2016), pp.1–23
Simmons, A. Chapter 11 Future Trends for the Sterilisation of Biomaterials and Medical Devices A. In Sterilisation of biomaterials and medical devices; 2016; pp 1–23.
B.J. Parsons, Chapter 3 Sterilisation of Healthcare Products by Ionising Radiation: Principles and Standards (Woodhead Publishing Series in Biomaterials, In Sterilisation of biomaterials and medical devices, 2016), pp.1–23
I. Anderson, 3D Print Addit. Manuf. 4(2), 110–115 (2017). https://doi.org/10.1089/3dp.2016.0054
B. Wittbrodt, J.M. Pearce, The effects of PLA color on material properties of 3-D printed components. Addit. Manuf. (2015). https://doi.org/10.1016/j.addma.2015.09.006
O.A. Mohamed, S.H. Masood, J.L. Bhowmik, Adv. Manuf. (2015). https://doi.org/10.1007/s40436-014-0097-7
A. Melocchi, F. Parietti, A. Maroni, A. Foppoli, A. Gazzaniga, L. Zema, Int. J. Pharm. 509(1–2), 255–263 (2016). https://doi.org/10.1016/j.ijpharm.2016.05.036
D. Puppi, F. Chiellini, Appl. Mater. Today (2020). https://doi.org/10.1016/j.apmt.2020.100700
ASTM/ISO 52900. Additive Manufacturing - General Principles - Terminology. ASTM Int. 2021, 2021 (II), 1–14.
Bae, C.; Diggs, A. B.; Ramachandran, A. Quantification and Certification of Additive Manufacturing Materials and Processes. In Additive Manufacturing: Materials, Processes, Quantifications and Applications; Elsevier Inc., 2018; pp 181–213. https://doi.org/10.1016/B978-0-12-812155-9/00006-2.
A. Shafiee, A. Atala, Trends Mol. Med. 22(3), 254–265 (2016). https://doi.org/10.1016/j.molmed.2016.01.003
U. Jammalamadaka, K. Tappa, J Funcl Biomater. (2018). https://doi.org/10.3390/jfb9010022
J.Y. Lee, J. An, C.K. Chua, Appl. Mater. Today 7, 120–133 (2017). https://doi.org/10.1016/j.apmt.2017.02.004
A. Youssef, S.J. Hollister, P.D. Dalton, Biofabrication (2017). https://doi.org/10.1088/1758-5090/aa5766
Advances in 3D Printing & Additive Manufacturing Technologies; Wimpenny, D. I., Pandey, P. M., Kumar, L. J., Eds.; Springer, 2017. https://doi.org/10.1007/978-981-10-0812-2.
A. Alafaghani, A. Qattawi, M.A. Ablat, Open J. Appl. Sci. 07(06), 291–318 (2017). https://doi.org/10.4236/ojapps.2017.76024
N.A. Sears, D.R. Seshadri, P.S. Dhavalikar, E. Cosgriff-Hernandez, Tissue Eng. Part B Rev. (2016). https://doi.org/10.1089/ten.teb.2015.0464
Choi, A. J.; Lu, Y.; Wicker, R. B. Additive Manufacturing: Innovations, Advances, and Application; 2016.
W. Wu, R. Cheng, J. Das Neves, J. Tang, J. Xiao, Q. Ni, X. Liu, G. Pan, D. Li, W. Cui et al., Advances in biomaterials for preventing tissue adhesion. J. Control. Release (2017). https://doi.org/10.1016/j.jconrel.2017.06.020
P. Ahangar, M.E. Cooke, M.H. Weber, D.H. Rosenzweig, Current biomedical applications of 3d printing and additive manufacturing. Appl. Sci. (2019). https://doi.org/10.3390/app9081713
Electrospinning Process and Nanofiber Research; Haghi, A. K., Zaikov, G. E., Eds.; Nova Science Publishers, Inc., 2011.
R. Stepanyan, A. Subbotin, L. Cuperus, P. Boonen, M. Dorschu, F. Oosterlinck, M. Bulters, Fiber diameter control in electrospinning. Appl. Phys. Lett. (2014). https://doi.org/10.1063/1.4900778
A. Ghilan, A.P. Chiriac, L.E. Nita, A.G. Rusu, I. Neamtu, V.M. Chiriac, Trends in 3D printing processes for biomedical field: opportunities and challenges. J. Polym. Environ. 28(5), 1345–1367 (2020). https://doi.org/10.1007/s10924-020-01722-x
M.A. Elsawy, K.-H. Kim, J.-W. Park, A. Deep, Hydrolytic degradation of polylactic acid (PLA) and its composites. Renew. Sustain. Energy Rev. (2017). https://doi.org/10.1016/j.rser.2017.05.143
N.G. Tanikella, B. Wittbrodt, J.M. Pearce, Tensile strength of commercial polymer materials for fused filament fabrication 3D printing. Addit. Manuf. 15, 40–47 (2017). https://doi.org/10.1016/j.addma.2017.03.005
K. Kalia, A. Ameli, Understanding the process-microstructure-property relationships in material extrusion additive manufacturing of polylactic acid microcellular foams. Addit. Manuf. (2023). https://doi.org/10.1016/j.addma.2023.103636
Hart, K. Fracture performance of annealed additely manufactured polymer structures fabricated with novel multi-material filaments; Army Research Laboratory, USA 2018.
S. Hou, T. Li, Z. Jia, L. Wang, Mechanical properties of sandwich composites with 3d-printed auxetic and non-auxetic lattice cores under low velocity impact. Mater. Des. 160(November), 1305–1321 (2018). https://doi.org/10.1016/j.matdes.2018.11.002
Ravi, P. Towards the Fabrication of Bioresorbable Constructs with Customized Properties Using Additive Manufacturing, 2017.
Ö. Keleş, C.W. Blevins, K.J. Bowman, Effect of build orientation on the mechanical reliability of 3D printed ABS. Rapid Prototyp. J. 23(2), 320–328 (2017). https://doi.org/10.1108/RPJ-09-2015-0122
C. Koch, L. Hulle, Van; Rudolph, N., Investigation of mechanical anisotropy of the fused filament fabrication process via customized tool path generation. Addit. Manuf. 16, 138–145 (2017). https://doi.org/10.1016/j.addma.2017.06.003
S.O. Akande, K. Dalgarno, J. Munguia, S.O. Akande, Process Control Testing for Fused Filament Fabrication. (2017). https://doi.org/10.1108/RPJ-07-2015-0084
F. Lederle, F. Meyer, G.-P. Brunotte, C. Kaldun, E.G. Hübner, Improved mechanical properties of 3D-printed parts by fused deposition modeling processed under the exclusion of oxygen. Prog. Addit. Manuf. 1(1–2), 3–7 (2016). https://doi.org/10.1007/s40964-016-0010-y
Harpool, T. D. Observing the Effects of Infill Shapes on the Tensile Characteristics of 3D Printed Plastic Parts. 2016, No. December, 88.
F. Kabirian, B. Ditkowski, A. Zamanian, R. Heying, M. Mozafari, An innovative approach towards 3D-printed scaffolds for the next generation of tissue-engineered vascular grafts. Mater. Today Proc. 5(7), 15586–15594 (2018). https://doi.org/10.1016/j.matpr.2018.04.167
S.F. Khan, H. Zakaria, Y.L. Chong, M.A.M. Saad, K. Basaruddin, Effect of infill on tensile and flexural strength of 3D printed PLA parts. IOP Conf. Ser. Mater. Sci. Eng. (2018). https://doi.org/10.1088/1757-899X/429/1/012101
J. Maloch, E. Hnátková, M. Žaludek, Effect of processing parameters on mechanical properties of 3D printed samples. Mater. Sci. Forum 919, 230–235 (2018). https://doi.org/10.4028/www.scientific.net/MSF.919.230
G. Gomez-gras, R. Jerez-mesa, J.A. Travieso-rodriguez, J. Lluma-fuentes, Fatigue Performance of Fused Filament Fabrication PLA Specimens. Mater. Des. 140, 278–285 (2018). https://doi.org/10.1016/j.matdes.2017.11.072
J.R.C. Dizon, A.H. Espera, Q. Chen, R.C. Advincula, Mechanical Characterization of 3D-Printed Polymers. Addit. Manuf. 20, 44–67 (2018). https://doi.org/10.1016/j.addma.2017.12.002
J.M. Chacón, M.A. Caminero, E. García-Plaza, P.J. Núñez, Mater. Des. 124, 143–157 (2017). https://doi.org/10.1016/j.matdes.2017.03.065
M. Ahmed, M. Islam, J. Vanhoose, M. Rahman, 3D Print Addit. Manuf. 4(2), 105–109 (2017). https://doi.org/10.1089/3dp.2016.0057
Abbas, D.; Mohammad Othman, D.; Basil Ali, H.; Author, C. Effect of Infill Parameter on Compression Property in FDM Process. Int. J. Eng. Res. andApplication www.ijera.com2017, 7 (December), 16–19. https://doi.org/10.9790/9622-0710021619.
S.F.S. Shirazi, S. Gharehkhani, M. Mehrali, H. Yarmand, H.S.C. Metselaar, N. Adib Kadri, N.A.A. Osman, Sci. Technol. Adv. Mater. 16(3), 1–20 (2015). https://doi.org/10.1088/1468-6996/16/3/033502
J.E. Pickett, D.J. Coyle, Polym. Degrad. Stab. (2013). https://doi.org/10.1016/j.polymdegradstab.2013.04.001
A.J. Dunbar, E.R. Denlinger, M.F. Gouge, T.W. Simpson, P. Michaleris, Addit. Manuf. 15, 57–65 (2017). https://doi.org/10.1016/j.addma.2017.03.003
R.K. Bordia, S.-J.L. Kang, E.A. Olevsky, J. Am. Ceram. Soc. 100(6), 2314–2352 (2017). https://doi.org/10.1111/jace.14919
X. Wang, B.O.A. Botchway, Y. Zhang, J. Yuan, X. Liu, Front. Mol. Neurosci. 12(April), 1–10 (2019). https://doi.org/10.3389/fnmol.2019.00081
S.M. Oskui, G. Diamante, C. Liao, W. Shi, J. Gan, D. Schlenk, W.H. Grover, Environ. Sci. Technol. Lett. (2016). https://doi.org/10.1021/acs.estlett.5b00249
N.A. Chartrain, C.B. Williams, A.R. Whittington, A Review on Fabricating Tissue Scaffolds Using Vat Photopolymerization. Acta Biomater. (2018). https://doi.org/10.1016/j.actbio.2018.05.010
M. Gholipourmalekabadi, A.M. Seifalian, A.M. Urbanska, M.D. Omrani, J.G. Hardy, Z. Madjd, S.M. Hashemi, H. Ghanbarian, P.B. Milan, M. Mozafari et al., 3D Protein-Based Bilayer Artificial Skin for the Guided Scarless Healing of Third-Degree Burn Wounds in Vivo. Biomacromol 19, 2409–2422 (2018). https://doi.org/10.1021/acs.biomac.7b01807
A. Garkal, D. Kulkarni, S. Musale, T. Mehta, P. Giram, Electrospinning Nanofiber Technology: A Multifaceted Paradigm in Biomedical Applications. New J. Chem. 45(46), 21508–21533 (2021). https://doi.org/10.1039/d1nj04159b
Kchaou, M.; Alquraish, M.; Abuhasel, K.; Abdullah, A.; Ali, A. A. Electrospun Nanofibrous Scaffolds: Review of Current Progress in the Properties and Manufacturing Process, and Possible Applications for Covid-19. Polymers (Basel). 2021, 13 (6). https://doi.org/10.3390/polym13060916.
Oliva, A. I. Morphological and Mechanical Properties of Electrospun Polycaprolactone Scaffolds : Effect of Applied Voltage. 2021, 1–15.
M. Herrero-Herreo, Role of Electrospinning Parameters on Poly ( Lactic-Co-Glycolic. Polymers (Basel). 13(695), 1–11 (2021)
McCullen, S. D.; Culbreath, C. J.; Vaughn, M. A.; Taylor, M. S. US10888407B2 Method for Forming a Multi-Layer Construct, 2021.
H. He, Y. Wang, B. Farkas, Z.K. Nagy, K. Molnar, Analysis and Prediction of the Diameter and Orientation of AC Electrospun Nanofibers by Response Surface Methodology. Mater. Des. (2020). https://doi.org/10.1016/j.matdes.2020.108902
A. Haider, S. Haider, I.K. Kang, A Comprehensive Review Summarizing the Effect of Electrospinning Parameters and Potential Applications of Nanofibers in Biomedical and Biotechnology. Arab. J. Chem. 11(8), 1165–1188 (2018). https://doi.org/10.1016/j.arabjc.2015.11.015
H.T. Zhang, Q. Wang, Z. Chen, F.L. Wei, IEEE Trans. Control Syst. Technol. 25(2), 611–618 (2017). https://doi.org/10.1109/TCST.2016.2557224
Yuan, H.; Zhou, Q.; Zhang, Y. Improving Fiber Alignment during Electrospinning. Electrospun Nanofibers 2017, 125–147. https://doi.org/10.1016/B978-0-08-100907-9.00006-4.
L. Palangetic, N.K. Reddy, S. Srinivasan, R.E. Cohen, G.H. McKinley, C. Clasen, Polymer (Guildf). 55(19), 4920–4931 (2014). https://doi.org/10.1016/j.polymer.2014.07.047
C. Enrique, G. Garcia, M. Rinaudo, Polymers (Basel). 13(1037), 1–20 (2021)
Jeffries, E. M. Biomimetic Micropatterning of Electrospun Scaffolds for Tissue Engineering, University of Pittsburgh, 2014.
K. Kataria, A. Gupta, G. Rath, R.B. Mathur, S.R. Dhakate, Int. J. Pharm. 469(1), 102–110 (2014). https://doi.org/10.1016/j.ijpharm.2014.04.047
J.C. Rodriguez, A.H. Chen, D.E. Carney, Burn. Open 6(4), 173–176 (2022). https://doi.org/10.1016/j.burnso.2022.09.002
Duckworth, J. Where Are All the Electrospun Medical Devices? https://www.electrospinning.co.uk/wp-content/uploads/2022/06/John-Duckworth_-TERMIS-22-.pdf. 2022.
Rafiei, M.; Jooybar, E.; Abdekhodaie, M. J.; Alvi, M. Construction of 3D Fibrous PCL Scaffolds by Coaxial Electrospinning for Protein Delivery. Mater. Sci. Eng. C 2020, 113 (March). https://doi.org/10.1016/j.msec.2020.110913.
Xie, X.; Chen, Y.; Wang, X.; Xu, X.; Shen, Y.; Khan, A. ur R.; Aldalbahi, A.; Fetz, A. E.; Bowlin, G. L.; El-Newehy, M.; et al. Electrospinning Nanofiber Scaffolds for Soft and Hard Tissue Regeneration. J. Mater. Sci. Technol. 2020, 59, 243–261. https://doi.org/10.1016/j.jmst.2020.04.037.
I.J.H. Barrientos, G.R. MacKenzie, C.G. Wilson, D.A. Lamprou, P. Coats, Biological Performance of Electrospun Polymer Fibres. Materials. (2019). https://doi.org/10.3390/ma12030363
Qamar Khan, M.; Kharaghani, D.; Khatri, Z.; Kim, I. S. Nanofibers for Medical Textiles. Handb. Nanofibers 2019, 887–904. https://doi.org/10.1007/978-3-319-53655-2_57.
Advances in Technical Nonwovens; Kellie, G., Ed.; Woodhead Pub., 2016.
O. Yousefzade, R. Katsarava, J. Puiggalí, Biomimetic Hybrid Systems for Tissue Engineering. Biomimetics 5(4), 1–31 (2020). https://doi.org/10.3390/biomimetics5040049
T.D. Brown, P.D. Dalton, D.W. Hutmacher, Melt Electrospinning Today: An Opportune Time for an Emerging Polymer Process. Prog. Polym. Sci. 56(56), 116–166 (2016). https://doi.org/10.1016/j.progpolymsci.2016.01.001
Yuan, H.; Zhou, Q.; Li, B.; Bao, M.; Lou, X.; Zhang, Y. Direct Printing of Patterned Three-Dimensional Ultrafine Fibrous Scaffolds by Stable Jet Electrospinning for Cellular Ingrowth. Biofabrication 2015, 7 (4). https://doi.org/10.1088/1758-5090/7/4/045004.
Sensini, A.; Gualandi, C.; Focarete, M. L.; Belcari, J.; Zucchelli, A.; Boyle, L.; Reilly, G. C.; Kao, A. P.; Tozzi, G.; Cristofolini, L. Multiscale Hierarchical Bioresorbable Scaffolds for the Regeneration of Tendons and Ligaments. Biofabrication 2019, 11 (3). https://doi.org/10.1088/1758-5090/ab20ad.
McCullen, S. D. Development, Characterization, and Function of Electrospun Nanocomposites for Tissue Engineerng. 2006.
A.R. D’Amato, M.T.K. Bramson, D.T. Corr, D.L. Puhl, R.J. Gilbert, J. Johnson, Solvent retention in electrospun fibers affects scaffold mechanical properties. Electrospinning. (2018). https://doi.org/10.1515/esp-2018-0002
V. Chausse, E. Casanova-Batlle, C. Canal, M.P. Ginebra, J. Ciurana, M. Pegueroles, Solvent-cast direct-writing and electrospinning as a dual fabrication strategy for drug-eluting polymeric bioresorbable stents. Addit. Manuf. 2023(71), 103568 (2022). https://doi.org/10.1016/j.addma.2023.103568
N.W. Pensa, A.S. Curry, P.P. Bonvallet, N.F. Bellis, K.M. Rettig, M.S. Reddy, A.W. Eberhardt, S.L. Bellis, 3D printed mesh reinforcements enhance the mechanical properties of electrospun scaffolds. Biomater. Res. 23(1), 1–7 (2019). https://doi.org/10.1186/s40824-019-0171-0
ISO 13485 Medical Devices; 2016.
ASTM. F1925: Standard Specification for Semi-Crystalline Poly(Lactide) Polymer and Copolymer Resins for Surgical Implants. ASTM Stand. 2022. https://doi.org/10.1520/F1925-09.
ASTM. F2902: Assessment of Absorbable Polymeric Implants. ASTM Stand. 2016, i, 1–13. https://doi.org/10.1520/F2902.
Chromatography, G. Standard Practice for Determination of Volatiles in Polymers by Static Headspace. Practice 2001, 14 (Reapproved), 4–7. https://doi.org/10.1520/D4526-96R07.2.
ASTM International, ASTM D638–14 Standard Test Method for Tensile Properties of Plastics. ASTM Int. 08, 46–58 (2003). https://doi.org/10.1520/D0638-14.1
ASTM D5034–21 Standard Test Method for Breaking Strength and Elongation of Textile Fabrics (Grab Test). 2021. https://doi.org/10.1520/D5034-09R17.2.
ASTM International. ASTM F1635 - 11 Standard Test Method for in Vitro Degradation Testing of Hydrolytically Degradable Polymer Resins and Fabricated Forms for Surgical Implants. 2011, i, 1–7. https://doi.org/10.1520/F1635-11.2.
ISO 10993 Biological Evaluation of Medical Devices - Part 1 Evaluation and Testing within a Risk Management Process.
3D Systems to Expand Healthcare Applications Portfolio with Acquisition of Kumovis https://www.3dsystems.com/press-releases/3d-systems-expand-healthcare-applications-portfolio-acquisition-kumovis.
History of (Osteopore) Development https://www.osteopore.com/history.html.
L.E. Visscher, H. Phuc, M.A. Knackstedt, D.W. Hutmacher, 3D Printed Polycaprolactone Scaffolds with Dual Macro-Microporosity for Applications in Local Delivery of Antibiotics. Mater. Sci. Eng. C 87(January), 78–89 (2018). https://doi.org/10.1016/j.msec.2018.02.008
Nyberg, E.; Rindone, A.; Dorafshar, A.; Grayson, W. L. Comparison of 3D-Printed Poly-e-Caprolactone Scaffolds Functionalized with Tricalcium Phosphate, Hydroxyapatite, Bio-Oss, or Decellularized Bone Matrix. https://doi.org/10.1089/ten.tea.2016.0418.
Dutta Roy, T.; Simon, J. L.; Ricci, J. L.; Rekow, E. D.; Thompson, V. P.; Parsons, J. R. Performance of Degradable Composite Bone Repair Products Made via Three-Dimensional Fabrication Techniques. J. Biomed. Mater. Res. - Part A 2003, 66 (2), 283–291. https://doi.org/10.1002/jbm.a.10582.
D. Drummer, S.C. Cuéllar, D. Rietzel, D. Rietzel, Suitability of PLA / TCP for Fused Deposition Modeling. (2012). https://doi.org/10.1108/13552541211272045
Hutmacher, D. W. Scaffold Design and Fabrication Technologies for Engineering Tissues — State of the Art. 2001, 12 (1), 107–124.
M. Bartnikowski, C. Vaquette, S. Ivanovski, Workflow for Highly Porous Resorbable Custom 3D Printed Scaffolds Using Medical Grade Polymer for Large Volume Alveolar Bone Regeneration. Clin. Oral Implants Res. 31(5), 431–441 (2020). https://doi.org/10.1111/clr.13579
Zhai, C.; Wang, J.; (Paul) Tu, Y.; Chang, G.; Ren, X.; Ding, C. Robust Optimization of 3D Printing Process Parameters Considering Process Stability and Production Efficiency. Addit. Manuf. 2023, 71 (December 2022), 103588. https://doi.org/10.1016/j.addma.2023.103588.
I. Valizadeh, T. Tayyarian, O. Weeger, Influence of Process Parameters on Geometric and Elasto-Visco-Plastic Material Properties in Vat Photopolymerization. Addit. Manuf. 72(June), 103641 (2023). https://doi.org/10.1016/j.addma.2023.103641
A.J. Guerra, J. Ciurana, 3D-Printed Bioabsordable Polycaprolactone Stent: The Effect of Process Parameters on Its Physical Features. Mater. Des. 137, 430–437 (2018). https://doi.org/10.1016/j.matdes.2017.10.045
J. Ivorra-Martinez, L. Quiles-Carrillo, D. Lascano, S. Ferrandiz, T. Boronat, Effect of Infill Parameters on Mechanical Properties in Additive Manufacturing. Dyna 95(4), 412–417 (2020). https://doi.org/10.6036/9674
J. Zhang, F. Meng, E. Ferraris, Temperature Gradient at the Nozzle Outlet in Material Extrusion Additive Manufacturing with Thermoplastic Filament. Addit. Manuf. 73(June), 103660 (2023). https://doi.org/10.1016/j.addma.2023.103660
B. Akhoundi, M. Nabipour, F. Hajami, D. Shakoori, An Experimental Study of Nozzle Temperature and Heat Treatment (Annealing) Effects on Mechanical Properties of High-Temperature Polylactic Acid in Fused Deposition Modeling. Polym. Eng. Sci. 60(5), 979–987 (2020). https://doi.org/10.1002/pen.25353
J. Wang, H. Xie, Z. Weng, T. Senthil, L. Wu, A novel approach to improve mechanical properties of parts fabricated by fused deposition modeling. Mater. Des. 105, 152–159 (2016). https://doi.org/10.1016/j.matdes.2016.05.078
W. Ahmed, S. Siraj, A.H. Al-Marzouqi, Embracing additive manufacturing technology through fused filament fabrication for antimicrobial with enhanced formulated materials. Polymers (Basel). 13(9), 1523 (2021). https://doi.org/10.3390/polym13091523
S.L. Sing, W.Y. Yeong, Polymers (Basel). 13(1098), 13–14 (2021)
Electrospinning: From Basic Research to Commercialization, Soft Matte.; Kny, E., Ghosal, K., Thomas, S., Eds.; The Royal Society of Chemistry, 2018. https://doi.org/10.1039/9781788010580-FP007.
A.J. Guerra, P. Cano, M. Rabionet, T. Puig, J. Ciurana, Polym. Adv. Technol. 29(8), 2327–2335 (2018). https://doi.org/10.1002/pat.4344
Acknowledgements
The authors would like to thank and acknowledge support from the Clemson Advanced Material Research Lab (AMRL) Microscopy Center and all of our colleagues at Poly-Med, Inc.
Funding
Open access funding provided by the Carolinas Consortium.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
CJC, MST, and SDM are all employed by Poly-Med, Inc., a producer of bioresorbable materials.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Culbreath, C.J., Taylor, M.S., McCullen, S.D. et al. A Review of Additive Manufacturing in Tissue Engineering and Regenerative Medicine. Biomedical Materials & Devices (2024). https://doi.org/10.1007/s44174-024-00183-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44174-024-00183-3